Protein Glycosylation Analysis Service
Glycosylation is a critical post-translational modification that shapes protein function—yet its complexity often poses challenges in analysis. Variations in N-glycosylation (asparagine-linked) and O-glycosylation (serine/threonine-linked) can impact protein stability, therapeutic efficacy, and disease mechanisms. At Creative Biolabs, our protein glycosylation analysis service simplifies this process, delivering precise insights into glycosylation patterns. Whether you need to map modification sites, quantify glycan structures, or characterize heterogeneity, our services support advancements in biopharmaceuticals, diagnostics, and basic research.
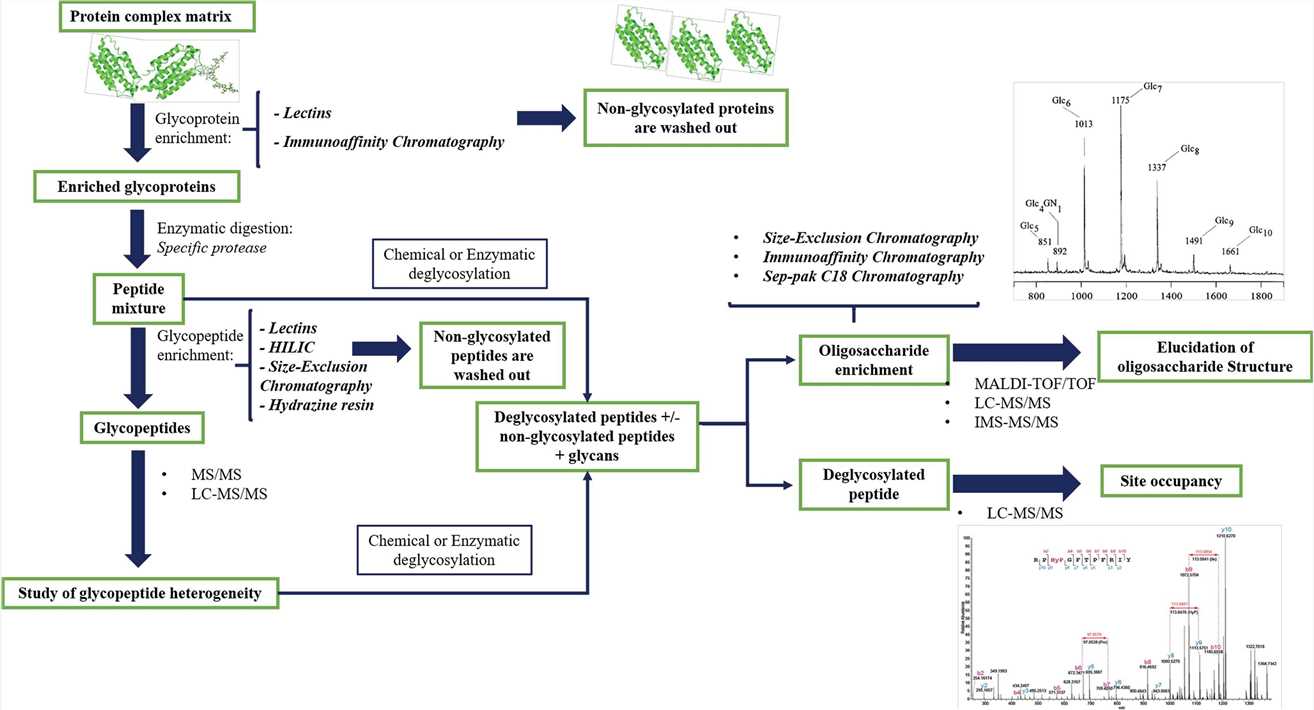 Fig.1 Workflow for mass spectrometry (MS)-based glycoprotein analysis.1
Fig.1 Workflow for mass spectrometry (MS)-based glycoprotein analysis.1
N-Glycosylation Analysis Solutions
Our N-glycosylation solutions combine accuracy and sensitivity:
-
Glycan Release:
Mild enzymatic release using PNGase F preserves protein integrity while isolating N-glycans. -
Enrichment:
Lectin affinity chromatography or HILIC selectively enriches glycopeptides, reducing interference from non-glycosylated proteins. -
Structural Analysis:
- Mass Spectrometry (MS/MS): Identifies monosaccharide composition, linkage types, and site-specific modifications (e.g., fucose, sialic acid).
- HPLC: Separates glycan isomers and quantifies glycoform ratios for heterogeneity analysis.
-
Site Occupancy:
Stable isotope labeling (SILAC/iTRAQ) or label-free methods measure glycosylation efficiency at each asparagine site.
O-Glycosylation Analysis Solutions
Tackling O-glycosylation heterogeneity with tailored strategies:
-
Site Mapping:
HILIC enrichment of O-glycopeptides, paired with high-resolution MS (e.g., Orbitrap), precisely locates serine/threonine modification sites. -
Glycan Characterization:
- Chemical Degradation (β-elimination): Releases O-glycans for fluorescent labeling (e.g., APTS) and sensitive detection by CE or MS.
- Lectin Microarrays: High-throughput screening for specific O-glycans (e.g., mucin-type glycans) without glycan release.
- Quantification: Label-free peak area analysis or isotope tagging adapts to low-abundance samples.
6-Stage Analysis Workflow: Step-by-Step Clarity with Quality Controls
-
Sample Prep:
Support for diverse samples (cell lysates, serum, tissue extracts) with preprocessing guidelines. -
Enrichment (Optional):
Lectin-based purification removes non-glycosylated proteins, achieving >95% enrichment efficiency. -
Glycan Release:
Enzymatic (PNGase F/Endo H) or chemical methods chosen to avoid artificial modifications. -
Separation & Detection:
- N-Glycosylation: HPLC-MS/MS identifies >100 glycoforms in a single run.
- O-Glycosylation: Capillary electrophoresis (CE) with fluorescent labeling detects down to fmol levels.
-
Data Analysis:
Bioinformatics teams decode glycan heterogeneity, site distribution, and modification frequency. -
Report Delivery:
Comprehensive report including raw data, glycoform maps, statistical analysis, and biological insights.
Sample Requirements
To ensure the best results, please provide samples as follows:
-
Protein Samples:
Pure proteins, cell lysates, serum, or plasma. -
Quantity:
At least 50 µL of sample with a concentration of 1 mg/mL or higher. For low - concentration samples, larger volumes may be needed. -
Storage:
Store samples at - 80°C and avoid freeze - thaw cycles. Ship with dry ice to maintain stability.
How Protein Glycosylation Powers Your Research & Development
Protein glycosylation isn't just a biological curiosity—it's a critical lever for advancing drug discovery, optimizing therapeutic efficacy, and unlocking precision in bioprocessing. For researchers and biotech professionals, understanding glycosylation isn't optional: it's the key to overcoming challenges in protein stability, disease biomarker discovery, and regulatory compliance. Our services bridge discovery to delivery, with workflows optimized for therapeutic development—from early-stage glycan profiling to late-phase batch consistency testing. Here's why it matters for your work—and how our services can accelerate your success.
Decode Protein Behavior to Drive Better Biology & Biotech
Glycosylation shapes over 50% of human proteins, influencing everything from folding to immune interactions (e.g., antibody Fc glycans that amplify ADCC activity by 50% when optimized). Our services help you:
- Optimize protein production: Identify glycosylation hotspots that stabilize therapeutic proteins (like darbepoetin alfa's extended half-life via added N-glycans), reducing aggregation and improving yield.
- Mimic natural function: Decode glycan-mediated trafficking tags (e.g., lysosomal enzymes' mannose-6-phosphate signals) to engineer cell-targeted therapies with precision.
- Enhance antibody design: Uncover how fucosylation or sialylation impacts effector functions—critical for developing next-gen cancer immunotherapies with maximized ADCC/CDC activity.
Turn Disease Glycosylation Signals into Actionable Insights
Aberrant glycosylation isn't just a marker of disease—it's a driver. From cancer (hyper-glycosylated MUC1 as an early ovarian cancer marker) to diabetes (IgG sialylation linked to insulin resistance), our analyses help you:
- Discover novel biomarkers: Leverage our high-sensitivity MS/MS to identify disease-specific glycoforms (e.g., galactose-deficient IgG in rheumatoid arthritis) for diagnostic development.
- Understand pathological mechanisms: Map glycosylation changes in Alzheimer's-associated β-amyloid or tau proteins to uncover aggregation triggers, guiding therapeutic target selection.
- Mitigate risk in bioprocessing: Detect non-human glycoforms (e.g., α-gal in CHO cells) that cause immunogenicity, ensuring your candidates meet strict regulatory standards.
Engineer Therapeutics with Glycosylation
Glycoengineering is transforming biopharma:
- Extend half-lives & potency: Our site-specific analysis helps you replicate darbepoetin alfa's success—adding glycan sites to reduce renal clearance and boost efficacy.
- Boost antibody therapy: Use our FUT8 knockout method to develop afucosylated antibodies with 2-3x stronger ADCC, like those targeting solid tumors.
- Design next-gen vaccines: Characterize HIV gp120-like glycopeptides to mimic conserved sugar epitopes, driving robust neutralizing antibody responses faster.
Aligning with Client Needs
Biopharmaceutical Clients
- Ensure therapeutic protein consistency (e.g., monoclonal antibodies) by optimizing N-glycosylation homogeneity and reducing immunogenic risks.
- Improve recombinant protein production: Analyze N-glycosylation site occupancy to enhance expression stability and in-vivo half-life.
Clinical Researchers
- Discover disease biomarkers: Identify aberrant O-glycosylation in mucins (e.g., MUC1 in cancer) for early diagnostic tool development.
- Optimize vaccine design: Decode glycan structures on viral glycoproteins (e.g., influenza hemagglutinin) to enhance immune responses.
Why Choose Us?
| Core Strengths | Client Value |
|---|---|
| Dual Glycosylation Coverage | One-stop analysis for N- and O-glycosylation, saving time and resources. |
| Low Sample Requirement | Start with just 50 µL serum or 100 µg protein—ideal for rare samples. |
| Regulatory Compliance | Data meets international standards for direct IND/BLA submissions. |
| Custom Solutions | Tailored protocols for complex samples (e.g., mucins, membrane glycoproteins). |
| Rapid Turnaround | Standard projects completed in 2–3 weeks; rush options available for urgent needs. |
Unlocking the insights of protein glycosylation is now straightforward. Whether you're developing biotherapeutics, investigating disease mechanisms, or characterizing novel glycoproteins, Creative Biolabs' protein glycosylation analysis service delivers the precision and clarity you need. Contact us today for a free customized plan, or explore our n-glycosylation analysis of protein and o-glycosylation analysis of protein services. Let's transform glycosylation complexity into actionable science—accelerate your research and development with our expertise.
Reference:
- Illiano, Anna, et al. "Protein glycosylation investigated by mass spectrometry: an overview." Cells 9.9 (2020): 1986. Distributed under Open Access license CC BY 4.0, without modification.https://doi.org/10.3390/cells9091986