Glycosylation Analysis Service
Introduction
Glycosylation influences almost everything: protein folding and stability, receptor engagement, pharmacokinetics, immune recognition, even higher-order structure. However, the same intricacy that gives glycosylation so much power also makes accurate measurement difficult. This is an area in which our team specializes. At Creative Biolabs, we transform glycans' invisible language into precise, actionable insights that expedite discovery, reduce development risk, and clarify structure-function links across biologics, cell systems, and emerging modalities. With decades of hands-on glyco-science experience and an industry-hardened CRO attitude, we combine best-in-class analytical technologies with purpose-built study design, guaranteeing that the data that matters is delivered correctly the first time. We provide extensive experience in glycomics and glycoproteomics, as well as strict quality control and exact reporting, to elucidate glycosylated antibodies and glycoproteins, glycolipids, and the rapidly developing field of glyco-conjugated RNAs.
Why is In-Depth Glycosylation Analysis Important?
In today's competitive research and development landscape, overlooking glycosylation is a risk you cannot afford to take. A comprehensive glycan profile is a critical quality attribute that impacts every stage of the product lifecycle, from initial discovery to lot-release testing.
- For therapeutic protein & antibody development, glycan structures directly control a biologic's efficacy, safety, and clinical performance.
- For biomarker discovery & diagnostics, unique glycan signatures on cells serve as powerful markers for early disease detection and prognosis.
- For vaccine development, characterizing microbial surface glycans is essential for designing immunogens that elicit a protective immune response.
- For fundamental biological research, analyzing glycans is critical to understanding core biological processes like cell recognition, signaling, and development.
What We Analyze
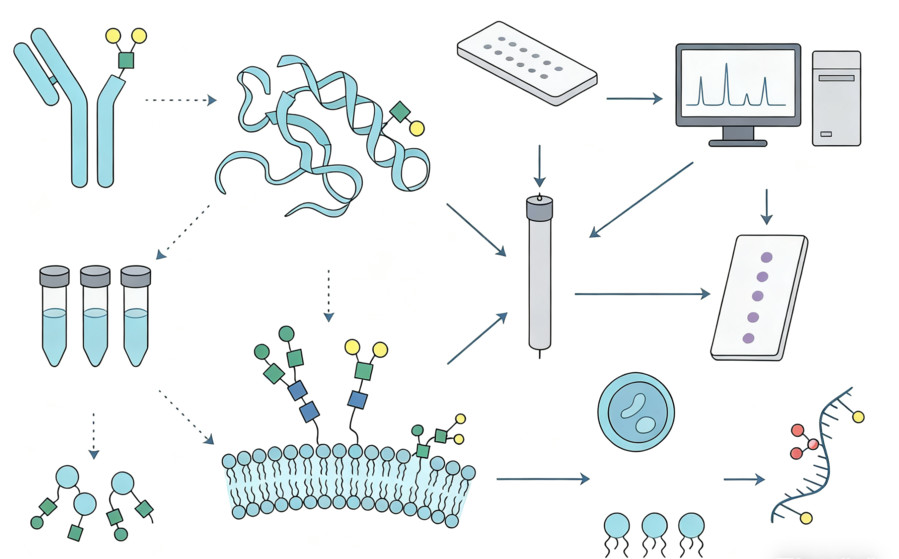
- N-glycans: high-mannose, hybrid, complex, sialylated and fucosylated variants, bisecting GlcNAc.
- O-glycans: Core 1–4, sialyl-T/Tn, extended structures and sulfated forms.
- Glycopeptides: site occupancy, site-specific microheterogeneity, relative quantitation.
- Intact proteins & subunits: glycoform envelopes, mass shifts, and higher-order distribution.
- Glycolipids: neutral glycosphingolipids and gangliosides (e.g., GM1, GM2, GM3, GD1a/b), ceramide heterogeneity.
- GlycoRNA: lectin-reactive and chemically tagged RNA subclasses enriched from cellular contexts for orthogonal confirmation.
| Track | Best suited for | Common decisions enabled |
|---|---|---|
| Antibody Glycosylation Analysis Service | mAbs, bispecifics, Fc-fusions | Potency vs. ADCC/CDC trade-offs, lot-to-lot control, process and clone selection |
| Protein Glycosylation Analysis Service | Enzymes, receptors, cytokines, complex biologics | Mechanism hypotheses, stability optimization, comparability studies |
| Glycolipid Analysis Service | Membrane extracts, brain tissue, cells | Pathway mapping, biomarker exploration, functional correlates |
| GlycoRNA Analysis Service | Cellular RNA fractions | Emerging biology insights, modality characterization, screening hypotheses |
Start Your Glycosylation Analysis Project
Glycosylation Analysis Service Overviews
Antibody Glycosylation Analysis Service
Monoclonal and engineered antibodies live or die by their Fc glycoform profiles. Afucosylation can boost ADCC; terminal galactose or sialic acid can fine-tune effector functions; high-mannose content can shift clearance. Our antibody-focused analytics make these levers visible and measurable—reliably, lot after lot. Our services include:
- Monoclonal Antibody Glycosylation Analysis Service
- Polyclonal Antibody Glycosylation Analysis Service
- Fab Glycosylation Analysis Service
- Fc Glycosylation Analysis Service
What you get
- Released N-glycan profiles with fluorescent labeling
- Site-specific Fc (and Fab, if present) glycopeptide maps by LC-MS/MS
- Intact/subunit mass for glycoform envelope confirmation
- Relative abundance tables for afucosylation, galactosylation, sialylation, bisecting GlcNAc
Protein Glycosylation Analysis Service
Outside antibodies, glycosylation remains a master regulator of protein behavior. We reveal site occupancy, site-specific microheterogeneity, and intact mass shifts across diverse proteins—from soluble enzymes to multi-domain receptors and fusion constructs. Our services include:
What you get
- Site-specific mapping of N- and O-glycans
- Released-glycan profiling for comprehensive compositional views
- Monosaccharide and linkage analysis where structural ambiguity remains
- Custom assays for sulfation, phosphorylation, or unusual features (where applicable)
Glycolipid Analysis Service
Glycolipids frame membrane architecture and mediate recognition at the cell surface. In neurobiology and immunology especially, small changes in ganglioside patterns can carry large functional consequences. Our glycolipid analytics deliver confident identification and relative quantitation across complex samples. Our services include:
- Glycosphingolipids Analysis Service
- Glycoglycerolipids Analysis Service
- Lipopolysaccharide Analysis Service
- Glycosylphosphatidylinositol Anchor Analysis Service
- Antigen-associated Glycolipid Analysis Service
What you get
- Ganglioside paneling
- Ceramide backbone profiling (chain length, saturation) to contextualize function
- Class-specific enrichment for sensitivity in challenging samples
GlycoRNA Analysis Service
Glyco-conjugated RNAs are an exciting and rapidly evolving frontier. Early evidence suggests subsets of cellular RNAs bear glycans that can be enriched by lectins or chemical tagging and confirmed by mass spectrometry. Because methods are still maturing, robust controls and orthogonal verification are essential. Our services include:
What you get
- Lectin-guided or chemical-tagging enrichments tailored to your biological question
- Multi-step cleanup to minimize matrix interferences
- MS-based confirmation and qualitative profiling of enriched RNA subclasses
- Control-first study design to rule out artifacts and ensure specificity
- Transparent limitations and next-step recommendations as the field evolves
How We Work with You
- We begin with your scientific question and the decisions it must support.
- We will then work with you to design a fit-for-purpose study. In this stage, we will align platforms, depth and controls according to the sample type and matrix constraints that you provide us with.
- Pilot and refine – a strategic approach that involves a swift and risk-averse pilot to assess the viability of the project and calibrate its sensitivity and dynamic range.
- Our system is designed to scale with confidence, expanding seamlessly to your full sample set with locked methods and predefined quality control gates.
- It is vital to ensure clarity. Our reports focus on the metrics required, with publication-quality figures and transparent methods.
Sample Types and Typical Starting Materials
We are flexible and pragmatic—send what makes sense, and we will right-size the method:
- Purified proteins (mAbs, bispecifics, Fc-fusions, enzymes, receptors)
- Complex matrices (cell lysates, conditioned media, serum/plasma, tissues)
- Membrane preparations and lipid extracts for glycolipid work
- RNA fractions prepared under RNase-controlled workflows for glycoRNA studies
Not sure how much to ship? Send us an inquiry before making decision.
Case Study: Mapping Viral Glycoproteins for Vaccine Design (SARS-CoV-2)
The spike (S) protein of the SARS-CoV-2 virus is the primary target for vaccines and therapeutic antibodies. The S protein is heavily glycosylated, and this "glycan shield" plays a crucial role in evading the host immune system and mediating viral entry into cells. To inform vaccine design, a comprehensive site-specific glycosylation analysis of the recombinant S protein was performed. Using advanced liquid chromatography-mass spectrometry (LC-MS), researchers mapped the N-glycan profile at each of the 22 potential glycosylation sites on the protein. The analysis revealed a complex mix of oligomannose and complex-type glycans, with specific sites showing distinct processing states. This detailed glycan map provided an unprecedented view of the spike protein's surface, helping to identify which regions are shielded by glycans and which epitopes are more accessible to antibodies. This structural insight is invaluable for designing vaccines that can elicit broadly neutralizing antibody responses.
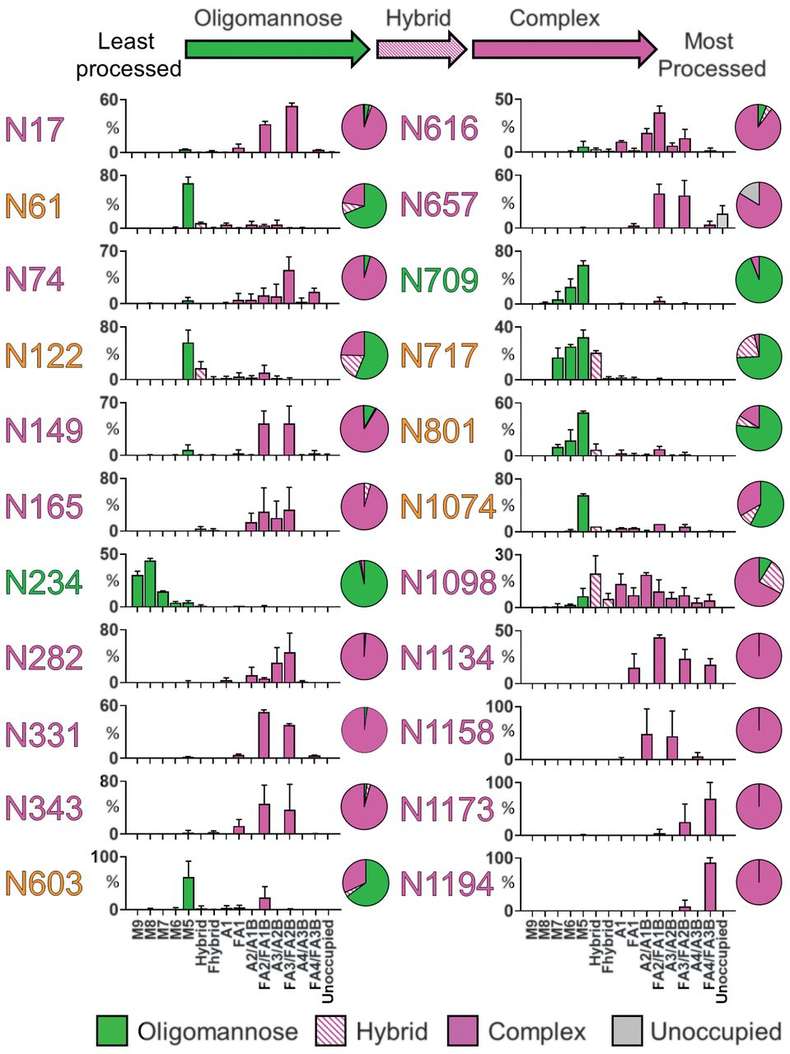 Fig.1 N-glycosylation sites on the SARS-CoV-2 spike protein.1
Fig.1 N-glycosylation sites on the SARS-CoV-2 spike protein.1
Whether you need a fast glycan fingerprint or a multi-layered analysis across proteins, lipids, and RNAs, our team is ready to help you pick the right depth at the right cost. Bring us your question, we will turn it into a clear plan and the data story your stakeholders can trust. Talk with our scientist now and turn complexity into clarity with Creative Biolabs' glycosylation analysis service.
FAQs
What sample types do you accept?
We accept purified proteins, antibodies, cell lysates, biofluids, tissues, cultured cells, glycolipid extracts, and RNA for GlycoRNA workflows.
Can you help me choose the right workflow for my question?
Absolutely. We start with a consultative scoping call to map hypotheses to methods: released-glycan profiling, site-specific glycoproteomics, intact glycoform analysis, glycolipid mapping, or glycoRNA assays. We recommend the minimal set that answers your question, then layer orthogonal confirmation only if it adds decision value, controlling cost and turnaround.
What are your sample shipping and stability recommendations?
Ship frozen on dry ice for proteins, antibodies, cells, and tissues; ship biofluids chilled if short transit is guaranteed, otherwise freeze. Avoid repeated freeze–thaw cycles and harsh detergents. Include buffer composition and any protease inhibitors. We provide pre-labeled kits, temperature loggers on request, and receipt QC to verify integrity before analysis begins.
How do you prevent artifacts like sialic-acid loss or O-acetyl migration during prep?
We use gentle release chemistries, low-pH stabilization, rapid derivatization when appropriate, and chilled workflows that minimize acid lability. For O-acetylated sialic acids, we employ conditions that retain modifications and produce diagnostic fragments. Controls and stress tests quantify any prep-induced changes, and we annotate confidence levels explicitly in your report.
Reference:
- Watanabe, Yasunori, et al. "Site-specific glycan analysis of the SARS-CoV-2 spike." Science 369.6501 (2020): 330-333. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1126/science.abb9983
Supports
- Understanding Glycosylation
- Glycosylation Influences Blood Type
- GM3 Antibody in Cancer Immunotherapy
- GM3 Ganglioside in Disease & Anti-GM3 Antibody Tools
- Engineering High-Affinity scFv for Next-Generation GD2-CAR-T Therapy
