CANOMAD Syndrome: A Neuropathy Driven by Anti-Ganglioside Antibodies
The Ganglioside: Key Targets in CANOMAD Syndrome
CANOMAD (Chronic Ataxic Neuropathy, Ophthalmoplegia, M-protein, and cold Agglutinins, with Disialosyl antibodies) syndrome is a rare autoimmune disorder defined as a chronic ataxic neuropathy associated with IgM monoclonal gammopathy. Its key characteristics include ophthalmoplegia, M-protein with cold agglutinin activity, and the presence of anti-disialosyl antibodies. The syndrome predominantly affects males around 50 years of age. The clinical presentation features prominent sensory ataxia, ophthalmoplegia, and absent tendon reflexes, with motor function remaining relatively preserved. Patients may also experience symptoms such as distal and perioral paresthesias, dysphagia, and dysarthria.
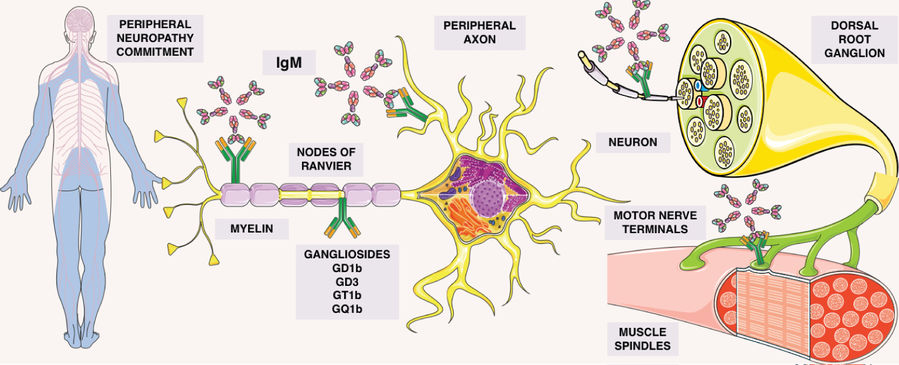 Fig.1 Gammopathy-mediated peripheral neuropathy: ganglioside-IgM interaction sites.1
Fig.1 Gammopathy-mediated peripheral neuropathy: ganglioside-IgM interaction sites.1
Serological hallmarks of the syndrome include IgM monoclonal gammopathy and antibodies targeting disialylated gangliosides such as GD3, GT1b, GD1b, and GQ1b, which are typically detected via ELISA. The pathogenic mechanism involves these antibodies binding to oligosaccharide chains on neural structures. This binding depletes gangliosides from nerve cell membranes, which in turn induces sensory neuron degeneration and demyelination. The specific profile of these anti-ganglioside antibodies is particularly significant, as their respective targets are highly expressed in different neural structures, including sensory neurons and nerve terminals. The targeting of these gangliosides is therefore closely linked to the syndrome's hallmark clinical features, such as sensory ataxia, by impairing proprioceptive signal transmission and disrupting membrane integrity and axonal function. Furthermore, cold agglutinins like anti-Pr2 may contribute to immune-mediated damage of the blood-brain barrier.
CANOMAD Syndrome Pathogenesis: How Anti-Ganglioside Antibodies Drive Nerve Damage
CANOMAD syndrome's onset ties to IgM antibodies mistakenly targeting disialylated gangliosides, key in neural tissues like nodes of Ranvier. These antibodies, from clonal B cells, latch onto gangliosides with disialosyl groups. Molecular mimicry, triggered by prior infections (e.g., Campylobacter jejuni), makes the immune system produce cross-reactive antibodies. Once bound, they activate complement pathways, causing nerve damage. This hits large sensory neurons (sensory ataxia) and cranial nerves (ophthalmoplegia), and may disrupt neuromuscular junctions. Some IgM acts as cold agglutinins, causing blood issues. Persistent antibody production, linked to B-cell disorders in some, drives the syndrome's chronic progression.
Where in the Nervous System Do Antibodies Bind?
Evidence from reviews points to several sites:
- Dorsal root ganglion (DRG) neurons
- Peripheral axons and myelin
- Nodes of Ranvier
- Muscle spindles
- Motor nerve terminals
Antibody binding at these locations provides a clear map for laboratory studies of conduction, signal stability, and sensorimotor control.
Who Is Affected and What Is the Usual Course?
Summaries of published cases suggest:
- More common in males, with average onset around the 5th decade of life.
- Heterogeneous presentation and relapsing patterns can occur over years.
These features underline the need for long-term data and careful tracking of antibody profiles in research.
What Do Scientists Measure?
- ELISA against disialosyl gangliosides (GD3, GT1b, GQ1b; GD1b) is standard in reports; many samples show elevated total IgM.
- Because anti-Pr is sialic-acid–dependent, sialidase (neuraminidase) controls help test whether binding truly depends on sialic acid.
RUO Services You Can Rely on
All services above are for laboratories only. We do not provide clinical testing or patient services.
- Analytical profiling (lipid class level):
-
Epitope-level mapping (array platforms):
- Glycolipid Microarray | Glycosphingolipid Microarray
- Sialoside Microarray (for disialosyl/sialic-acid dependence)
- Broad controls: Glycan Microarray, 100 Glycan Microarray, Oligosaccharide Microarray, Polysaccharide Microarray
-
Custom antibody generation (target-specific):
- Anti-Glycolipid Antibodies Development Services (GM1/GM2/GM3/GD1a/GD1b/GD2/GD3/GT1a/GT1b/GQ1b)
- Anti-Other Glycolipid Antibody Development (Anti-Sulfatide/Galactocerebroside/Globoside)
Published Data
CANOMAD Syndrome is a rare chronic-ataxic autoimmune neuropathy linked to IgM monoclonal gammopathy, primarily affecting men in their 50s (women may onset in their 40s). Its core symptoms include universal sensory ataxia, ophthalmoplegia, areflexia, and cranial nerve involvement—with limb motor function relatively preserved. Serologically, it's defined by IgM antibodies targeting disialosyl gangliosides (GD3, GT1b, GQ1b, GD1b) and often cold agglutinins like anti-Pr2; as shown in Figure 1, anti-Pr2 disrupts the blood-brain barrier by interacting with capillary endothelium, letting immune mechanisms access peripheral nervous system (PNS) myelin and enabling IgM adhesion to PNS structures. Diagnosis combines clinical features with ELISA-detected anti-ganglioside IgM, and nerve ultrasound aids by identifying demyelination-related nerve enlargement. For management, IVIg stabilizes symptoms via antibody neutralization, while rituximab is less effective—making early accurate diagnosis critical for optimal long-term care.
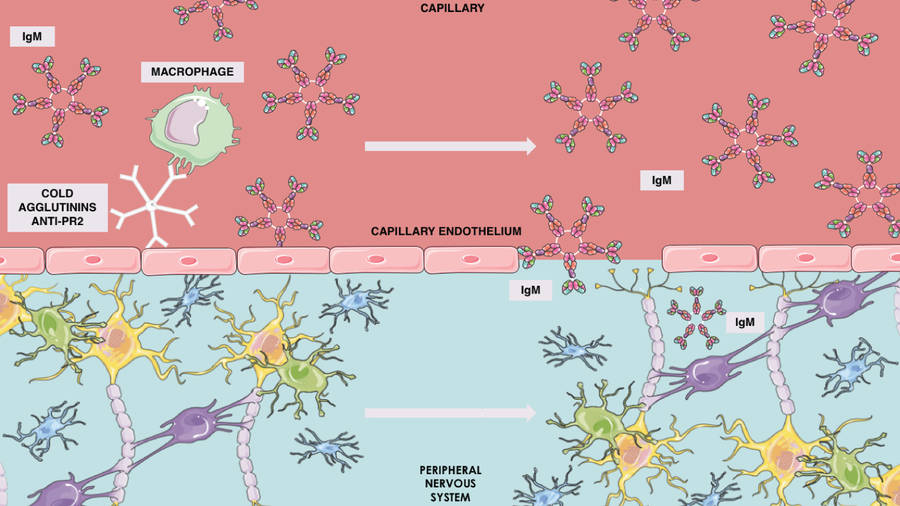 Fig.2 Cold agglutinins and IgM in CANOMAD syndrome.1
Fig.2 Cold agglutinins and IgM in CANOMAD syndrome.1
How Can Research Move Forward?
-
Standardize serology for GD3/GT1b/GD1b/GQ1b with explicit sialic-acid controls.
Sialoside Microarray, Glycolipid/Glycosphingolipid Microarrays. -
Bridge binding to function, adding complement readouts and node/NMJ-style models in your lab.
Glycolipid analysis service for standardized antigen prep; custom anti-ganglioside antibodies as reference reagents. - Ready-to-use anti-ganglioside antibodies
FAQs
What is CANOMAD and why are anti-ganglioside antibodies central to it?
CANOMAD is a rare, chronic neuropathy associated with IgM reactivity to disialosyl gangliosides. From a research standpoint, antibody–glycolipid interactions can illuminate mechanism and stratify preclinical models. We support laboratories with glycolipid analysis service and epitope-level mapping on glycolipid microarray and sialoside microarray to characterize binding patterns. All services are for laboratories only; no clinical testing or patient services.
How does CANOMAD differ from Guillain–Barré or Miller Fisher syndrome in study design?
Unlike GBS or Miller Fisher syndrome, which are typically acute and IgG-mediated (e.g., anti-GQ1b IgG), CANOMAD is chronic, relapse-prone, and often IgM-dominant with cold agglutinins and a monoclonal component. Study plans therefore emphasize longitudinal sampling, IgM-focused analytics, and disialosyl motif resolution, plus cold-sensitive preanalytical controls to preserve clinically relevant antibody activities in vitro.
What should laboratories focus on?
Clear serology panels (GD3, GT1b, GD1b, GQ1b), sialic-acid controls, and bridging from binding to complement activation and nerve-signal models. This helps connect what antibodies bind with what antibodies do.
What sample types can you process for analytical profiling?
We routinely handle research-grade sera, purified immunoglobulins, cell or tissue extracts, and glycolipid fractions. Lipid class–level profiling can be performed with glycolipid analysis service, glycosphingolipids analysis service, glycoglycerolipids analysis service, and GPI anchor analysis service.
Can you generate custom antibodies against specific CANOMAD-relevant gangliosides?
Yes—our anti-glycolipid antibodies development services cover GM1/GM2/GM3 and GD1a/GD1b/GD2/GD3/GT1a/GT1b/GQ1b, plus anti-sulfatide, anti-galactocerebroside, and anti-globoside options. Projects begin with microarray-guided epitope selection to reduce cross-reactivity risk. Generated antibodies are provided for laboratory research use, accompanied by characterization data aligned to your intended assays.
Reference:
- Rickli, Júlia Machado, Gabriela Pomaleski, and Marcus Vinícius Magno Gonçalves. "CANOMAD: A multi-faceted disease." (2020). Distributed under Open Access license CC BY, without modification. https://doi.org/10.23937/2378-3001/1410104
Supports
- Glycolipid
- GM3 Antibody in Cancer Immunotherapy
- GM3 Ganglioside in Disease & Anti-GM3 Antibody Tools
- Engineering High-Affinity scFv for Next-Generation GD2-CAR-T Therapy
