Custom Anti-sTn Antibody Development for Immuno-Oncology Research
In cancer, tumor cells employ a vast arsenal of tactics to survive and spread. One of their most effective strategies is camouflage. They cloak themselves in a dense layer of specific sugar molecules to hide from the immune system, promote metastasis, and resist therapy. Glycocalyx is a frontier of intense research in immuno-oncology. A key molecule in this malignant defense shield is the sialyl-Tn (sTn) antigen. At Creative Biolabs, we support cutting-edge immuno-oncology research with an advanced custom anti-TACA antibody development service and a catalog of validated anti-sTn antibody products and We build the precise tools researchers need to target the sialyl-Tn (sTn) antigen, a critical molecule in cancer's defense mechanisms.
Understanding the Thomsen-Friedenreich (TF) Antigen Family
The Tn antigen belongs to a group of closely related tumor-associated carbohydrate antigens (TACAs) called the Thomsen-Friedenreich (TF) antigen family. These are all "truncated O-glycans," meaning they are short sugar chains linked to a serine (Ser) or threonine (Thr) residue on a protein. Understanding this family is key to understanding the Tn antigen. The biosynthesis pathway is as follows:
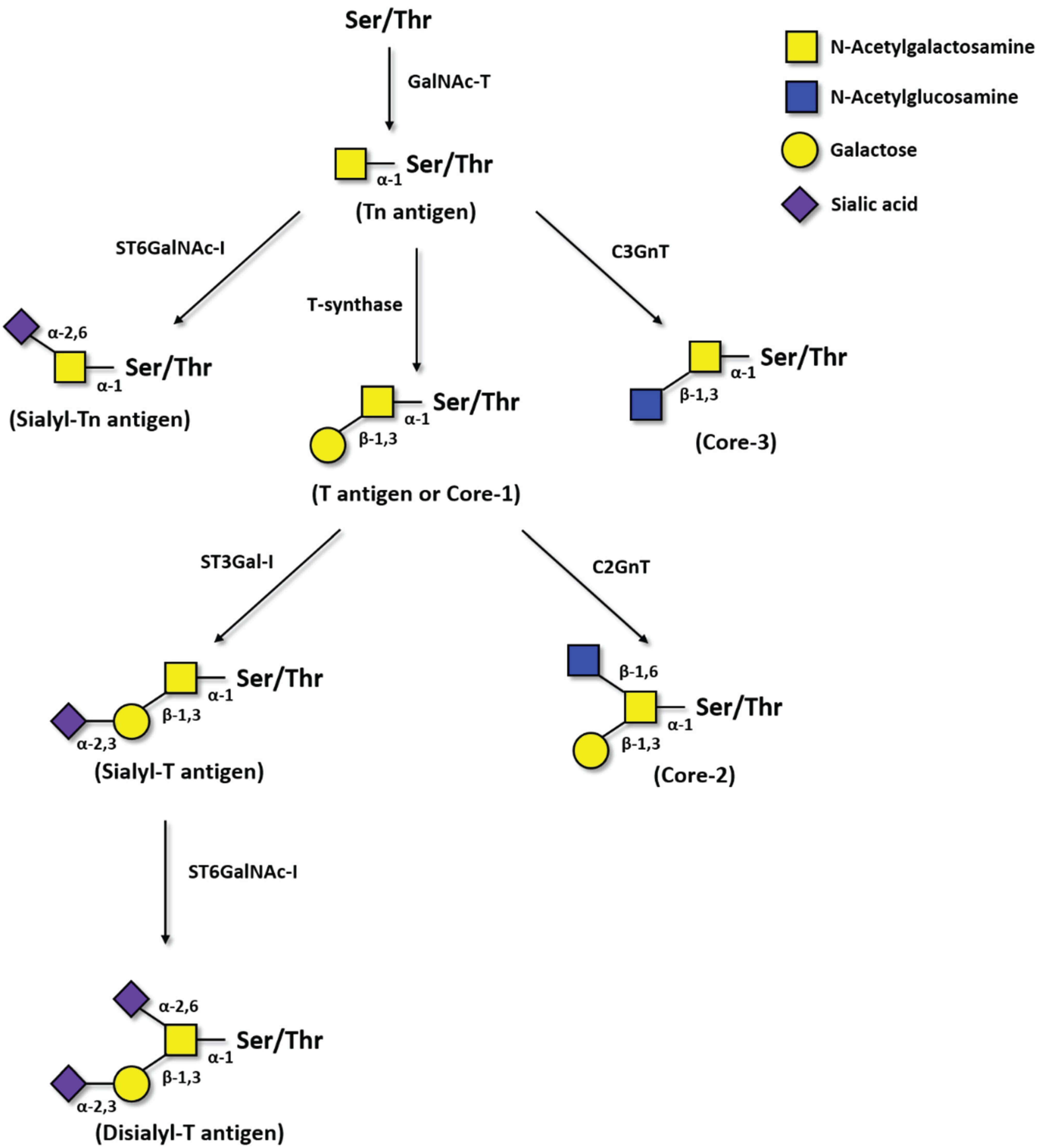 Fig.1 Biosynthesis pathway of Tn, sTn, T, and sT antigens.1
Fig.1 Biosynthesis pathway of Tn, sTn, T, and sT antigens.1
- The Start: Tn Antigen (CD175): The entire process begins when an enzyme (a GalNAc transferase) attaches a single N-acetylgalactosamine (GalNAc) sugar to a protein. This single sugar structure, GalNAc-O-Ser/Thr, is the Tn antigen. In healthy cells, it is an immediate precursor. (Know More about Custom Anti-Tn Antibody)
- Elongation to T Antigen: A critical enzyme called T-synthase (and its chaperone, Cosmc) immediately adds a galactose sugar to the Tn antigen. This creates the T antigen (Gal-GalNAc-O-Ser/Thr). The T antigen is also a precursor, which is then typically extended into longer, more complex chains (Core 1, Core 2, etc.).
-
Sialylation: Alternatively, other enzymes called sialyltransferases can add a sialic acid molecule to these truncated structures, creating "capped" antigens.
- If sialic acid is added to the T antigen, it becomes the sT (sialyl-T) antigen.
- If sialic acid is added directly to the Tn antigen, it becomes the sialyl-Tn (sTn) antigen (CD175s). The expression of sTn is a significant event in cancer progression and is a key focus for immuno-oncology research.
This entire group, including Tn, T, sTn, and sT, makes up the core TF antigen family. Each one represents a different failure point in the cell's glycosylation pathway, and each one provides a unique clue about the cancer's state.
The Functional Roles of sTn in Cancer
Because sTn is on the cell surface, is cancer-specific, and has clear functional roles in immune evasion and metastasis, it is one of the most promising targets in modern immuno-oncology research.
- Immune Evasion: This is its most critical role. The sTn antigen is a high-affinity ligand for Siglec receptors on immune cells. Binding of sTn to Siglec-7 (on NK cells) or Siglec-15 (on macrophages) effectively paralyzes these first-line defenders, allowing the tumor to grow unchecked.
- Metastasis: The sTn antigen helps cancer cells break away from the primary tumor and travel through the bloodstream. It interacts with selectin receptors on endothelial cells, enabling the cancer cell to adhere to the walls of blood vessels in distant organs, thereby forming new tumors.
- Chemoresistance: High sTn expression has been linked to resistance to standard chemotherapy drugs, making it a "multi-drug resistance" associated antigen.
Our Custom Anti-sTn Antibody Development Platform
Developing a therapeutic-grade anti-sTn antibody is a significant technical challenge. The antibody must bind only to the Neu5Acα2-6GalNAc structure. It absolutely must not cross-react with the unsialylated Tn antigen or any of the dozens of sialylated structures in the body. Our platform is engineered from the ground up to solve this specificity problem.
Phase 1: Strategic Antigen and Immunogen Design
This is the foundation of the entire project. We design synthetic glycopeptide antigens that present the sTn antigen in its natural context, often using a backbone from a protein like MUC1.
- Our synthetic antigens feature the precise α2-6 sialic acid linkage. This forces the immune response to focus on this specific structure, which is critical for distinguishing sTn from other sialylated glycans.
- The sTn-glycopeptide is conjugated to a carrier protein and combined with a potent adjuvant formulation to generate a robust, highly targeted immune response.
Phase 2: Antibody Generation and High-Throughput Screening
We offer multiple discovery engines to find the perfect antibody clone for your immuno-oncology application.
- Phage Display Library Screening: This is our preferred method for therapeutic discovery. We can screen our massive, fully human antibody libraries against the sTn target.
- Hyperdoma™ Platform: Hybridoma is the classic and robust method for generating high-quality mouse monoclonal antibodies, ideal for developing stable research tools.
Phase 3: Advanced Specificity and Functional Profiling
A binding antibody is not enough. For immuno-oncology, you need a functional antibody. Our screening cascade profiles for both specificity and function. Your anti-sTn antibody candidates are screened against a comprehensive panel to eliminate all cross-reactivity.
| Screening Assay | Target Molecule | Desired Result | Purpose |
|---|---|---|---|
| Positive Screen | sTn-glycopeptide (e.g., sTn-MUC1) | Strong Binding | Confirms recognition of the target. |
| Crucial Negative Screen | Tn-glycopeptide (e.g., Tn-MUC1) | No Binding | This is the top priority. Ensures no cross-reactivity with the precursor. |
| Negative Screen 2 | sT-glycopeptide | No Binding | Ensures no cross-reactivity with sialyl-T. |
| Negative Screen 3 | sLeA / sLeX Antigens | No Binding | Ensures no cross-reactivity with other common sialylated TACAs. |
Phase 4: Antibody Engineering, Production, and Validation
We deliver a final product that is ready for your experiments.
- For immuno-oncology projects, we can perform affinity maturation, humanization, and re-formatting (e.g., into a full-length IgG1, a scFv, or a bispecific antibody).
- We can produce your final, validated clone at any scale with high purity and low endotoxin levels suitable for in vivo studies.
Applications for Your Custom Anti-sTn Antibody
Your custom anti-sTn antibody will be a powerful tool for advancing immuno-oncology research:
- ADC Development: Use as the targeting arm to deliver a potent payload to sTn-positive tumors.
- CAR-T/NK Cell Research: Provide the binding domain for novel cell-based immunotherapies.
- Glyco-Checkpoint Blockade: Study the sTn-Siglec axis and test the antibody's ability to restore anti-tumor immunity, alone or in combination with traditional PD-1/CTLA-4 inhibitors.
Our Portfolio of Anti-sTn (CD175s) Antibody Products
To accelerate your advanced immuno-oncology research, Creative Biolabs provides a specialized portfolio of high-performance anti-sTn (CD175s) antibody products. These are not just standard detection reagents; they are research-grade tools specifically characterized for the demanding, functional applications that immuno-oncology requires. Our catalog features a range of monoclonal sialyl Tn antibody clones with verified specificity, ensuring they do not cross-react with the precursor Tn antigen. Using our validated anti-sTn reagents allows your team to bypass initial development hurdles and move directly to pivotal functional studies. We encourage you to explore our catalog to find the specific clones, isotypes, and formats that match your research goals.
-
Mouse Anti-Sialyl Tn Monoclonal Antibody (AGM-105YJ)(CAT#: AGM-105YJ)Online InquiryHost: MouseAntibody Isotype: IgG1, κSpecies Reactivity: HumanApplication: IHC, ELISA
-
Mouse Anti-Sialyl Tn Monoclonal Antibody (AGM-106YJ)(CAT#: AGM-106YJ)Online InquiryHost: MouseAntibody Isotype: IgG1Species Reactivity: HumanApplication: ICC, IF, IHC
-
Mouse Anti-Sialyl Tn Monoclonal Antibody (AGC-0425-QX468)(CAT#: AGC-0425-QX468)Online InquiryHost: MouseAntibody Isotype: IgG2a, κSpecies Reactivity: HumanApplication: FC, ELISA
The sTn antigen is one of the most exciting targets in immuno-oncology today, but it is also one of the most technically demanding. Success requires a deep understanding of glycobiology and an antibody development platform built to overcome the unique challenges of specificity and function. Stop searching for an off-the-shelf antibody that might work. Let us make a custom anti-sTn antibody that is precisely engineered and validated for your specific immuno-oncology application. Contact us today to speak with a scientist and begin designing your custom antibody project.
FAQs
What is the typical timeline for developing a custom anti-sTn antibody?
A typical project, from antigen design to delivering a validated antibody, takes several months. Hybridoma development typically ranges from 4 to 6 months, while phage display discovery can be faster. We will provide a detailed, customized timeline in your project proposal after our initial technical consultation.
How do you guarantee the antibody is specific to sTn and won't bind to the precursor Tn antigen?
This is the most critical challenge and our highest priority. We use a comprehensive screening panel, testing every antibody candidate against both the sTn target and the unsialylated Tn antigen. Only clones that exhibit a high affinity for sTn and show zero cross-reactivity with Tn are selected to move forward.
My research involves CAR-T cells. Can you provide the antibody sequence or an scFv fragment?
Absolutely. Our phage display platform is ideally suited for this purpose. We can isolate high-affinity binders directly as scFv fragments, which are the functional domains for CAR constructs. We will provide the sequence of the validated binder, ready for your downstream engineering into a CAR-T or CAR-NK cell line.
If I start with a mouse antibody, can you humanize it for later in vivo studies?
Yes, we offer a full suite of antibody engineering services. If we identify a promising mouse monoclonal antibody with ideal functional properties, our team can humanize it for you. We use advanced CDR grafting and computational modeling to maintain high affinity while minimizing immunogenicity for pre-clinical research.
How do we begin the process?
The best way to start is by contacting us for a technical consultation. One of our glycan antibody specialists will discuss your research goals and target applications with you. From there, we can outline a specific project strategy and provide a detailed, no-obligation proposal for your review and consideration.
Reference:
- Loureiro, Liliana R., et al. "Challenges in antibody development against Tn and Sialyl-Tn antigens." Biomolecules 5.3 (2015): 1783-1809. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/biom5031783
Supports
- TACAs Overview
- Guide to Blood Group Antigens
- Comparing sLeA and sLeX Roles in Cancer
- CA19-9 as a Pancreatic Cancer Biomarker
- Lewis Antigen System Overview
- TACA-Targeted ADCs, CAR-Ts, and RICs
