Custom Anti-sLeA (CA19-9) Antibody Development for Pancreatic Cancer Research
Pancreatic cancer remains one of the most significant challenges in modern oncology. Its aggressive nature, late-stage diagnosis, and resistance to standard therapies demand new research tools and novel therapeutic strategies. At the center of this challenge is a complex molecular target: sialyl-Lewis A (sLeA), the carbohydrate antigen better known as CA19-9 (Carbohydrate Antigen 19-9). For decades, CA19-9 has been used as a clinical biomarker. However, its proper biological role in tumor progression is a rapidly expanding field of study. Researchers now understand that sLeA is an active participant in cancer cell adhesion, metastasis, and immune evasion. To explore this critical target, you need tools of extraordinary precision. Standard, off-the-shelf antibodies often fall short when targeting complex glycans. This challenge is true for many crucial Tumor-Associated Carbohydrate Antigens (TACAs), from the complex epitopes on aberrant MUC1 glycans to sLeA itself. Creative Biolabs provides a comprehensive custom anti-TACA antibody development solution: a dedicated, end-to-end service for developing high-affinity, high-specificity custom anti-sLeA (CA19-9) antibodies. Partner with us to create the exact antibody you need to drive your pancreatic cancer research forward.
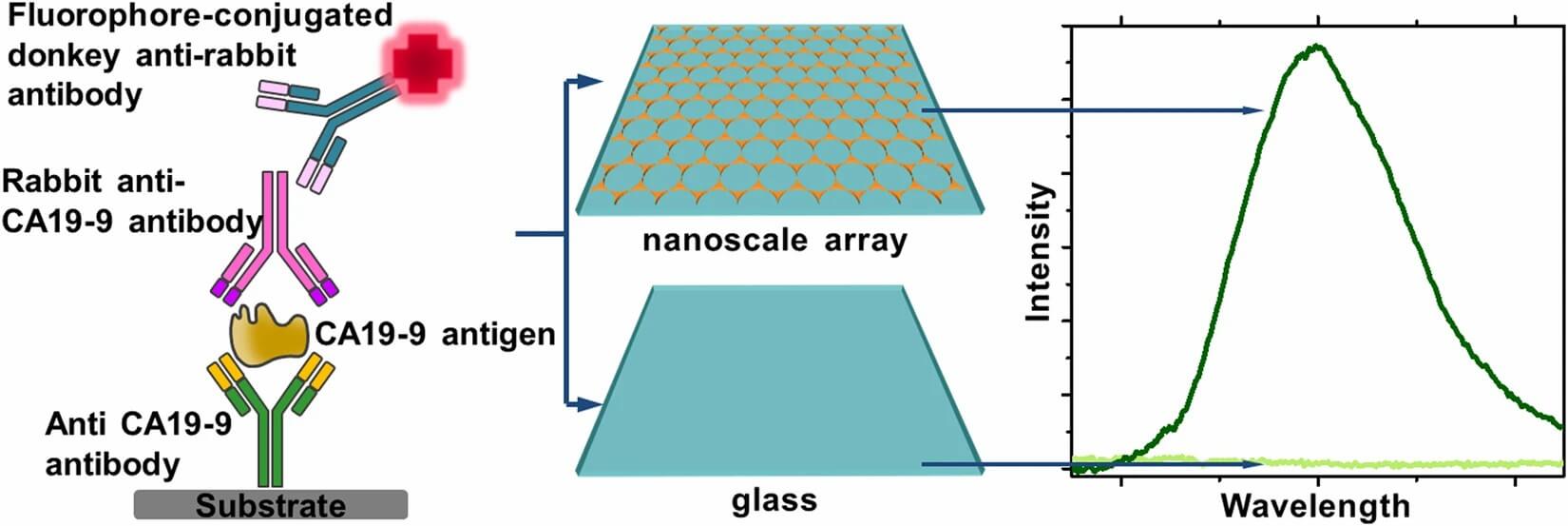 Fig.1 Near-infrared fluorescence immunoassay for CA 19-9 pancreatic cancer biomarker.1,2
Fig.1 Near-infrared fluorescence immunoassay for CA 19-9 pancreatic cancer biomarker.1,2
Understanding the Target: Sialyl-Lewis A (CA19-9)
What is Sialyl-Lewis A?
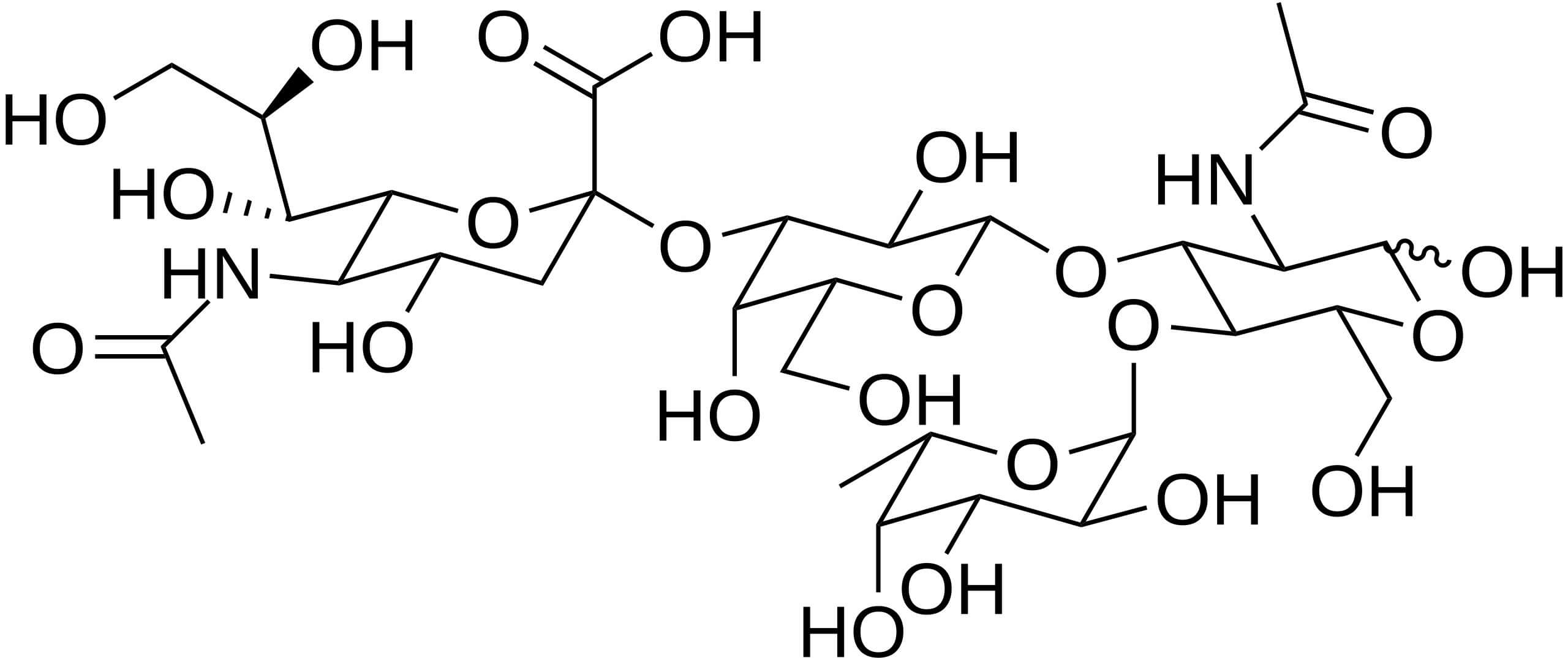 Fig.2 The structure of sialyl Lewis A (CA19-9).3
Fig.2 The structure of sialyl Lewis A (CA19-9).3
Sialyl-Lewis A (sLeA, CA19-9) is a glycan, or a complex carbohydrate structure. It is not a protein. This is a critical distinction, as developing antibodies against glycans requires specialized expertise. This specific glycan is found on the surface of cells, attached to either proteins (forming glycoproteins) or lipids (forming glycolipids). In healthy adult tissues, sLeA is expressed at very low levels, primarily in the pancreas, biliary ducts, and stomach lining. CA19-9 was first identified in the early 1980s by an antibody (1116-NS-19-9) developed against a human colon carcinoma cell line. Scientists discovered that this antibody recognized the sLeA glycan.
The Critical Role of sLeA (CA19-9) in Cancer Biology
Your research needs powerful tools because sLeA is a potent molecule in cancer. Its role extends far beyond being a simple biomarker.
A Defining Feature of Pancreatic Cancer
In pancreatic cancer (specifically pancreatic ductal adenocarcinoma, PDAC), the expression of CA19-9 is dramatically upregulated. The molecule is overexpressed on the surface of cancer cells and shed in large amounts into the bloodstream. This shedding is what makes it a useful (though imperfect) serum biomarker for monitoring disease progression. However, for researchers, the sLeA on the cell surface is the more interesting target. (Know more about CA19-9 as a Pancreatic Cancer Biomarker)
More Than a Marker: An Active Player in Malignancy
Why does pancreatic cancer produce so much sLeA? Research shows it provides a direct survival advantage to the tumor.
- Promoting Metastasis: sLeA is a ligand that binds to other molecules. Its primary binding partners are proteins called selectins found on the lining of blood vessels. This sLeA-selectin interaction allows circulating tumor cells to "stick" to blood vessel walls, a critical first step in escaping the bloodstream and forming new tumors (metastasis).
- Immune Evasion: The dense layer of glycans on a cancer cell, rich in sLeA, can physically shield the cell. It can hide protein-based antigens from the body's immune cells (like T-cells), helping the tumor grow undetected.
- Driving Tumor Growth: Binding of sLeA can trigger signaling pathways within the cancer cell that promote proliferation and resistance to cell death.
Relevance in Other Gastrointestinal Cancers
While most famous in pancreatic cancer, sLeA (CA19-9) overexpression is also a feature of other cancers, making it a valuable target for a broader field of study. These include:
- Bile duct cancer (cholangiocarcinoma)
- Colon cancer
- Stomach (gastric) cancer
The Challenge: Why Glycan Antibodies Are Hard to Make
If sLeA is such a great target, why isn't the market flooded with perfect antibodies? Targeting glycans is one of the most challenging tasks in antibody engineering. Here are reasons why a generic approach fails. You need a custom strategy, designed from the ground up, with a deep understanding of glycan chemistry and immunology.
Low Immunogenicity
Carbohydrates, unlike proteins, are very poor at triggering a strong immune response. An animal's immune system often ignores them, making it challenging to generate high-affinity antibodies using traditional methods.
Structural Similarity
The body is full of similar-looking glycans (e.g., sialyl-Lewis X, Lewis A, Lewis X). An antibody might bind to the wrong one. This cross-reactivity can ruin experiments and lead to false conclusions.
T-Cell Independent Antigens
Glycans typically do not activate T-cells. This means they do not produce a strong "memory" response, making it hard to achieve the high-affinity antibodies that result from standard protein immunization.
Heterogeneity
The sLeA glycan can be attached to many different proteins and lipids. The "context" in which it is presented can affect how an antibody binds.
Our Service: A Comprehensive Custom Antibody Platform
We overcome these challenges by combining cutting-edge technology with world-class glycobiology expertise. We offer a full-service pipeline, from antigen design to antibody production and validation, explicitly tailored to your research needs.
This is the most critical step. A successful project starts with the right antigen. We do not use a one-size-fits-all approach. We consult with you to design and produce the optimal sLeA immunogen for your goal. Options include:
- Synthetic glycan-carrier conjugates
- Custom-designed multivalent neoglycoproteins
- CA19-9-expressing whole cell immunogens
We offer multiple platforms to generate the specific antibody format you need.
| Technology | Description | Best For... |
|---|---|---|
| Hybridoma Development | The classic, gold-standard method for generating high-quality monoclonal antibodies. It produces stable, immortal cell lines for a limitless supply. | IHC, ELISA, Western Blot, and Flow Cytometry. Proven reliability. |
| Phage Display Platform | A powerful in vitro method. We screen vast libraries of antibody fragments (scFv, Fab) to screen for binders against the sLeA antigen. | Rapid development, highly specific binders, and generating recombinant antibodies without using animals. Ideal for therapeutic discovery. |
Our screening process is designed to eliminate low-affinity and cross-reactive clones from day one. This begins with a direct ELISA to identify binders to the sLeA-conjugate antigen, followed immediately by a counter-screening against the carrier protein to discard any non-specific hits. The crucial final stage is our key quality step: a glycan array specificity screening. Here, lead candidates are challenged against a comprehensive panel of related structures—including the sLeA target, common cross-reactants like sialyl-Lewis X, and precursors like Lewis A and Lewis X. Only antibodies demonstrating exquisite specificity for sLeA advance, and we provide you with the complete data so you can see the specificity for yourself.
We validate the final antibody clones for your specific application.
- ELISA: We confirm binding and can test for sensitivity.
- Western Blot (WB): We test against lysates from pancreatic cancer cell lines known to express CA19-9.
- Flow Cytometry (FACS): We confirm the antibody binds to sLeA on the live cell surface, which is critical for functional studies.
- Immunohistochemistry (IHC): We test on paraffin-embedded (FFPE) tissue sections from known positive pancreatic cancer tumors.
- Immunofluorescence (IF): We provide high-resolution imaging validation.
- More applications upon request
Once you approve a final clone, we scale up production to meet your research needs.
- Scale-up from milligrams to grams.
- Using Protein A/G purification for high-purity.
- Isotyping and purity confirmed by SDS-PAGE and HPLC.
- We can modify your antibody by creating Fab or F(ab')2 fragments, or labeling with biotin, enzymes (HRP, AP), or fluorescent dyes (FITC).
Why Partner with Us?
We are not just an antibody company. We are a team of biologists, immunologists, and chemists with a special focus on the most challenging targets, like sialyl-Lewis A.
- Expertise: We understand the unique chemistry and handling required for glycans.
- Customization: You are not locked into a rigid process. We adapt the project—antigen, host, platform—to your exact goal.
- Collaboration: You receive regular updates and all validation data. Our PhD-level project managers speak your language and are true scientific partners.
- Quality Control: Our rigorous, multi-stage screening process (especially our glycan array) ensures you receive an antibody that is specific and reliable.
Stop struggling with generic, unreliable reagents. To truly understand the role of sialyl-Lewis A in pancreatic cancer, you need a tool as precise and unique as the target itself. Let's build that tool together. Contact our expert team today for a free, no-obligation consultation about your project.
Related Services
FAQs
Targeting glycans like sLeA is notoriously tricky. How do you guarantee specificity against related structures like sLeX?
Specificity is our highest priority. Our screening process includes a comprehensive glycan array. We test all lead candidates against a broad panel including sLeX, Lewis A, and Lewis X. Only clones that demonstrate exquisite, specific binding to sLeA are advanced, and you receive all the data.
What information do I need to provide to start a project?
To begin, we need to understand your primary research goal. This includes your intended application and any specific requirements for host species or antibody format. Your application is critical as it dictates our antigen design and validation strategy.
What specific antigen strategies do you use for sLeA, since it's a carbohydrate?
Because carbohydrates alone are poorly immunogenic, we use advanced strategies. The most common is using high-purity, chemically synthesized sLeA glycans conjugated to immunogenic carrier proteins like KLH. We also design multivalent neoglycoproteins or use whole CA19-9-expressing cancer cell lines as immunogens.
Can I get an antibody explicitly validated for flow cytometry on live pancreatic cancer cells?
Yes, application-specific validation is a core part of our service. If your goal is to conduct flow cytometry assays, we will screen and validate the final antibody clones using live pancreatic cancer cell lines known to express high levels of surface CA19-9, such as BxPC-3 or CAPAN-1.
Can you develop matched pairs of anti-sLeA antibodies for a sandwich ELISA kit?
Absolutely. We specialize in developing matched pairs. Our process involves screening our antibody library to identify two distinct antibodies that can bind to sLeA simultaneously. We then validate their function as a capture and detection pair, ensuring high sensitivity and specificity for your ELISA.
References:
- Alarfaj, Nawal Ahmad, Maha Farouk El-Tohamy, and Hesham Farouk Oraby. "CA 19-9 pancreatic tumor marker fluorescence immunosensing detection via immobilized carbon quantum dots conjugated gold nanocomposite." International journal of molecular sciences 19.4 (2018): 1162. https://doi.org/10.3390/ijms19041162
- Distributed under Open Access license CC BY 4.0, without modification.
- Distributed under Public Domain, from Wiki, without modification.
Supports
- TACAs Overview
- Guide to Blood Group Antigens
- Comparing sLeA and sLeX Roles in Cancer
- CA19-9 as a Pancreatic Cancer Biomarker
- Lewis Antigen System Overview
- TACA-Targeted ADCs, CAR-Ts, and RICs
