Fab Glycosylation Analysis Service
Antibody glycosylation, particularly in the Fab region, is an emerging frontier in therapeutic antibody engineering. While Fc glycosylation has long been recognized for its role in mediating effector functions through Fcγ receptor interactions and FcRn recycling, the Fab domain's N-linked glycosylation—present in approximately 15–25% of circulating IgGs—has now been implicated in modulating antigen binding, half-life, immunogenicity, and even structural conformation. Creative Biolabs offers a specialized Fab glycosylation analysis service using state-of-the-art mass spectrometric profiling to support biopharmaceutical R&D, biosimilar development, and immunology studies.
Why Focus on Fab Glycosylation?
Fab glycosylation can occur in the variable regions of either light or heavy chains, frequently introduced by somatic hypermutation. These modifications often escape traditional expression system quality checks but can profoundly influence antibody characteristics:
- Glycans near CDRs can alter epitope accessibility and affinity via steric or electrostatic effects.
- Sialylated or high-mannose Fab glycans can modulate serum half-life.
- Glycosylation may affect immune complex formation, aggregation, or effector function.
- Molecular dynamics simulations reveal altered interdomain motion and Fc exposure due to Fab glycans.
Our Step-by-Step Fab Glycosylation Analytical Workflow
Creative Biolabs implements a validated, high-throughput mass spectrometric protocol optimized for Fab glycan profiling in polyclonal or monoclonal antibodies
Step 1: IgG Capture and Domain Separation
Antibodies are isolated from serum, plasma, or purified sources using Fc-specific affinity beads. IdeS protease is then applied to specifically cleave below the hinge region, yielding separate Fab and Fc fragments without disrupting glycosylation.
Step 2: Glycan Release and Stabilization
Each domain is subjected to denaturing PNGase F digestion to release N-linked glycans. Sialic acid residues are ethyl-esterified (α2,6) or lactonized (α2,3) to preserve linkage information and stabilize labile structures.
Step 3: Glycan Purification
Released glycans are enriched and desalted via miniaturized hydrophilic interaction chromatography (HILIC) using cotton-thread SPE tips. This ensures removal of contaminants and enhances detection sensitivity without sample loss.
- Supports high-throughput, parallel processing
- Suitable for high-mannose, hybrid, and complex-type glycans
Step 4: Mass Spectrometry & Data Analysis
Glycans from Fab and Fc domains are profiled separately using MALDI-TOF-MS in reflectron positive mode. Internal calibrants and automated integration scripts allow detection of ≥37 glycoforms. Optional tandem MS enables structural validation. Side-by-side comparison with Fc glycans and across sample groups is included in deliverables.
Sample Requirements & Deliverables
To ensure accessibility and minimal sample burden, our service is designed for micro-scale input while maintaining high-resolution outputs:
| Sample Type | Amount Required | Other Requirements |
|---|---|---|
| Purified IgG | ≥ 20 μg |
|
| Serum or Plasma (human/mouse) | ≥ 5 μL | |
| Hybridoma Supernatant | ≥ 100 μL | |
| Recombinant Antibodies | ≥ 20 μg |
Clients receive a comprehensive report including:
- Glycan composition per domain (Fab vs. Fc)
- Quantitative trait analysis (e.g., sialylation linkage, fucosylation, bisection)
- Comparative plots and heatmaps
- Interpretation in context of Fc function or therapeutic implication
Why Choose Creative Biolabs?
With expertise in antibody glycomics and deep integration of mass spectrometry, Creative Biolabs offers a unique service tailored for Fab glycan profiling with:
- Ultralow sample requirement
- Domain-specific resolution (Fab vs. Fc)
- Linkage-specific sialic acid identification
- High-level reproducibility
Published Data
Fab glycosylation changes a lot during pregnancy. Scientists looked at IgG glycosylation during pregnancy and after giving birth. They saw that Fab had more monosialylation and fucosylation after delivery, but less disialylation. Bisection also went up a lot after birth. Fc, on the other hand, had less galactosylation and monosialylation after delivery. During pregnancy, Fab didn't change much, but Fc had more noticeable changes. These findings are important because they show that Fab glycosylation is very active during pregnancy, and it might play a big role in how our immune system works. This suggests that studying Fab glycosylation could help us understand more about pregnancy and maybe even find new ways to treat related health issues.
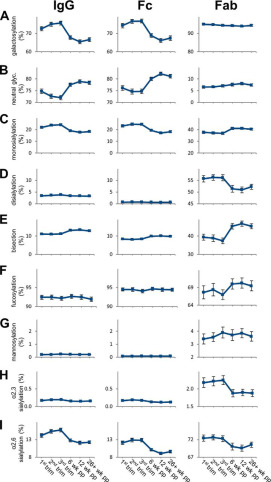 Fig.1 Pregnancy-associated fab glycosylation changes.1
Fig.1 Pregnancy-associated fab glycosylation changes.1
Related Services
Custom Glycosylation of Antibodies
Glycosylation is no longer a passive structural feature—it's a tunable determinant of antibody efficacy. At Creative Biolabs, our custom glycosylation of antibodies service allows precise engineering of N- or O-glycan patterns on monoclonal antibodies using cell line modulation, enzymatic remodeling, or chemoenzymatic strategies. Whether optimizing Fc effector functions (e.g., ADCC/CDC) or introducing Fab glycans to modulate antigen binding and half-life, our platform supports tailored glycoform enrichment, fucose or sialic acid incorporation, and bisecting GlcNAc modulation. End applications include biosimilar optimization, immune evasion studies, and PK engineering. Deliverables include glycan maps, functional readouts, and clone stability data.
Glycan Profiling Service
Our glycan profiling service offers an end-to-end solution for characterizing oligosaccharide structures on therapeutic proteins, antibodies, or glycoproteins. Utilizing LC-ESI-MS/MS, MALDI-TOF-MS, and fluorescent labeling, we identify and quantify N- and O-linked glycans with high resolution and sensitivity. Applications range from lot-to-lot consistency testing to disease biomarker discovery. Coupled with database-driven spectral matching, we report key glycosylation traits (e.g., galactosylation, fucosylation, sialylation, bisection) across samples or time points. This service is ideal for quality control, biosimilar comparability, and upstream glycoengineering evaluation.
Glycosylation Site Analysis
Site occupancy and microheterogeneity are central to therapeutic antibody function. Our glycosylation site analysis service pinpoints the exact amino acid residue(s) bearing N- or O-linked glycans using advanced LC-MS/MS glycopeptide mapping. After enzymatic digestion, glycopeptides are enriched and fragmented to reveal site-specific compositions and heterogeneity. We provide sequence coverage, glycan occupancy ratios, and identification of noncanonical glycosylation motifs (e.g., Fab or hinge region). This service is ideal for assessing host expression effects, mapping engineered glycosites, and ensuring regulatory compliance for biologics.
FAQs
References:
- Bondt, Albert, et al. "Immunoglobulin G (IgG) Fab glycosylation analysis using a new mass spectrometric high-throughput profiling method reveals pregnancy-associated changes." Molecular & Cellular Proteomics 13.11 (2014): 3029-3039. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1074/mcp.M114.039537
- van de Bovenkamp, Fleur S., et al. "The emerging importance of IgG Fab glycosylation in immunity." The Journal of Immunology 196.4 (2016): 1435-1441. https://doi.org/10.4049/jimmunol.1502136
- Saporiti, Simona, et al. "In silico evaluation of the role of Fab glycosylation in cetuximab antibody dynamics." Frontiers in Immunology 15 (2024): 1429600. https://doi.org/10.3389/fimmu.2024.1429600