100 Glycan Microarray
Introduction
Glycans' structural diversity and non-template-based biosynthesis make systematic analysis challenging. To address this, Creative Biolabs offers a 100 glycan microarray platform: a high-throughput, precision-engineered tool enabling parallel screening of glycan-binding proteins, antibodies, pathogens, or live cells across structurally diverse glycan ligands. By combining standardized array chemistry, stringent quality control, and MS-compatible workflows, our platform accelerates discovery in glycomics, immunology, oncology, and infectious disease research.
What Is the 100 Glycan Microarray?
The 100 glycan microarray is a solid-phase platform containing 100 well-characterized, structurally defined glycans immobilized onto a functionalized glass surface. These glycans cover a broad range of structural classes:
- Monosaccharides: Glucose, galactose, fucose, etc.
- Disaccharides and oligosaccharides: LacNAc, sialylated structures
- N-linked and O-linked motifs: Bi-, tri-, tetra-antennary glycans
- Pathogen-associated glycans: Lewis antigens, blood group determinants
Why Glycan Arrays Matter?
Glycan arrays offer a transformative approach for analyzing glycan–protein interactions with precision and scalability. By enabling simultaneous screening against a structurally diverse library of glycans—including N-/O-glycans, glycolipids, and glycosaminoglycans—these arrays facilitate high-throughput profiling of lectins, antibodies, pathogens, and even live cells. Their application spans immunology, cancer, infection biology, and glycoengineering. With Creative Biolabs' robust microarray platform, researchers can gain deep insights into glycan-binding specificity and affinity, accelerating basic research development.
Technology Overview: The 100 Glycan Microarray
Creative Biolabs' 100 glycan microarray is engineered using state-of-the-art slide surface chemistry and covalent glycan immobilization to ensure uniformity, orientation, and accessibility. Each slide features 100 distinct glycan structures printed in replicates to enhance statistical robustness.
Key Platform Specifications
- Creative Biolabs' 100 glycan microarray features 100 distinct glycan structures covering diverse categories such as N-/O-glycans, GAGs, and microbial motifs.
- Each glycan is printed in replicates (typically 3–4 spots) to ensure statistical reliability across the entire array.
- Our platform accommodates various biomolecular samples, including purified lectins, antibodies, viral particles, bacterial lysates, and live cells.
- The array is compatible with both fluorescence-labeled detection and label-free imaging methods to meet different sensitivity requirements.
- Creative Biolabs delivers comprehensive data outputs, including binding intensity maps, heatmaps, and clustering plots to facilitate advanced analysis.
- All results adhere to international standards, ensuring publication-ready, high-reproducibility data.
Structural Diversity in the 100 Glycan Panel
The microarray encompasses a balanced selection of mammalian, microbial, and synthetic glycans. This broad diversity facilitates application in a wide range of research domains.
| Example Glycan Structures | Applications | |
|---|---|---|
| N-glycans | Bi-antennary, tri-antennary, sialylated | Therapeutic antibody analysis, cancer markers |
| O-glycans | Core 1/2, Tn, STn | Mucin biology, tumor glycosylation |
| Fucosylated forms | Lewis X, Sialyl-Lewis X, H-antigen | Immunity, selectin-binding studies |
| GAG fragments | Heparan sulfate, chondroitin sulfate | ECM–receptor binding, virus-host interaction |
| Microbial glycans | LPS core motifs, capsular polysaccharides | Vaccine design, pathogen recognition |
| Glycolipids | GM1, GM2, Gb3 | Neurobiology, toxin binding, gangliosides |
Workflow of the 100 Glycan Microarray Assay
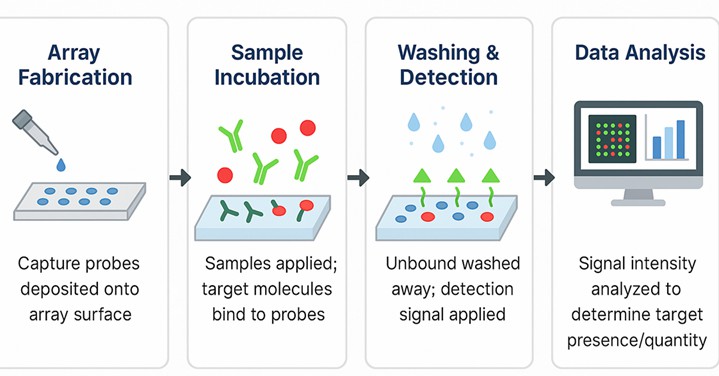 Fig.1 The 100 glycan microarray workflow at Creative Biolabs.
Fig.1 The 100 glycan microarray workflow at Creative Biolabs.
1. Array Fabrication
Glycans with reactive linkers are printed in replicates on a glass slide under controlled humidity. Slide surfaces ensure minimal background noise.
2. Sample Incubation
Samples containing GBPs (lectins, antibodies, viruses, or cells) are incubated with the array under optimized conditions. Binding occurs in a glycan-specific manner.
3. Washing & Detection
After incubation, non-specific interactions are removed through a series of gentle washes. Detection is then carried out via fluorophore-conjugated secondary antibodies, directly labeled analytes, or label-free platforms, depending on assay design.
4. Data Analysis
Fluorescence intensities are scanned, normalized, and analyzed to yield binding profiles, dissociation constants, and interaction heatmaps.
Deliverables & Samples: What You Need to Know
The glycan binding heatmap is a powerful visual tool provided as part of your project report when using the 100 glycan microarray service at Creative Biolabs. It displays interaction intensities between your submitted samples and the 100 distinct glycan structures on the array.
- X-axis (horizontal) represents individual glycan structures, each assigned a unique code (e.g., Glycan_1 to Glycan_100). These structures include a broad range of N-glycans, O-glycans, GAGs, microbial glycans, and others.
- Y-axis (vertical) lists your experimental samples—these can be purified proteins (e.g., lectins, antibodies), viral particles, bacterial lysates, or even fluorescently labeled live cells.
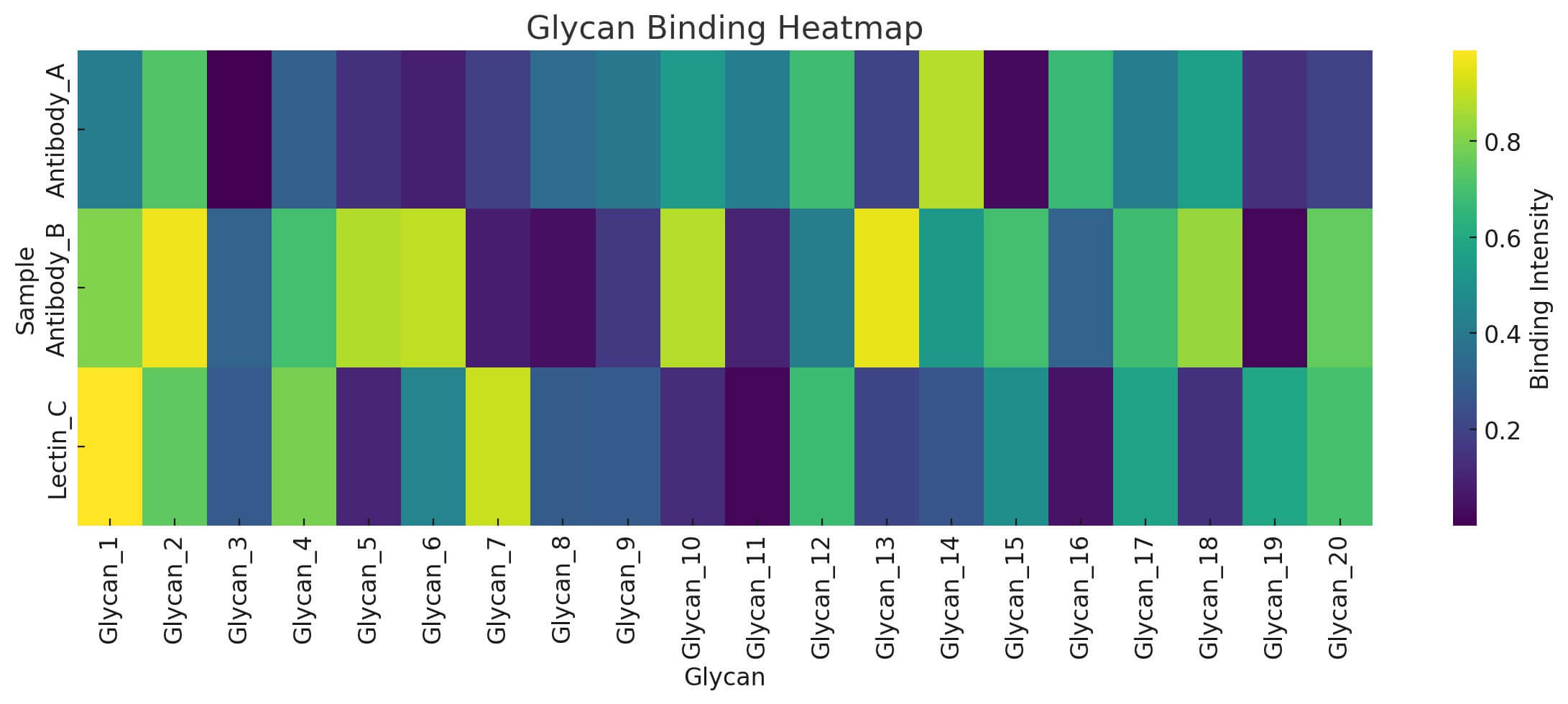 Fig.2 The schematic of glycan binding heatmap.
Fig.2 The schematic of glycan binding heatmap.
How to Submit Your Sample
Clients may submit:
- Protein samples (≥20 μg, ≥0.1 mg/mL; recommended buffer: PBS or Tris-based)
- Cell suspensions (10⁶–10⁷ cells per mL, labeled or unlabeled)
- Bacterial/viral preparations (minimum OD₆₀₀ = 0.5 for bacteria; titer >10⁶ PFU/mL for viruses)
Our technical team will assist with labeling strategies based on your molecule type and desired detection sensitivity.
Interpreting the Heatmap
Each cell in the heatmap reflects the relative fluorescence intensity (RFI) of your sample's binding to a specific glycan:
- Brighter colors indicate stronger binding affinity, suggesting a biologically relevant interaction.
- Low or no signal suggests weak or no detectable interaction.
You'll also receive binding profiles, raw signal intensities, replicate statistics, and clustering plots, enabling downstream functional insights—such as epitope mapping, receptor-ligand specificity, or pathogen adhesion profiling. Creative Biolabs' scientific team is always available to guide you in interpreting your data and correlating glycan-binding patterns with biological mechanisms or therapeutic potential.
Representative Applications
| Application | Description | Sample Type |
|---|---|---|
| Antibody Glycan Binding | Characterize antibody specificity toward tumor-associated glycans or vaccine-induced glycan epitopes | Human sera, monoclonal antibodies |
| Lectin Profiling | Assess endogenous lectin preferences for sialylation, fucosylation, etc. | Recombinant lectins, cell lysates |
| Virus-Glycan Interaction | Determine glycan receptors for influenza, coronavirus, norovirus, etc. | Intact viral particles |
| Cell Binding Assays | Evaluate cell surface glycan-binding profiles | Live cells, immune subsets |
Related Services You May Be Interested in
| Service We Provide | Service Description |
|---|---|
| 100 N-Glycan Microarray | Focused array for analyzing N-linked glycan interactions, useful in biopharmaceutical development |
| Oligosaccharide Microarray | Custom oligosaccharide profiling for synthetic, plant, or dietary glycans |
| Polysaccharide Microarray | Ideal for complex polysaccharide interaction studies, including microbial capsular and dietary fibers |
| Glycosaminoglycan (GAG) Microarray | For detailed investigation of ECM–protein and heparin-binding protein interactions |
| ABH-Glycan Microarray | Designed to analyze blood group antigen interactions, relevant in transfusion and pathogen studies |
| Human Milk Oligosaccharide Microarray | Specialized platform to study HMOs in nutrition, immunity, and gut microbiota modulation |
| Sialic Acid Microarray | Targets interactions involving diverse sialylated glycans, key in immunology and infection |
Why Choose Creative Biolabs?
- Decades of expertise in glycomics
- Fully customizable glycan arrays (up to 600 glycans)
- Flexible sample formats, including pathogen samples under BSL-2 conditions
- End-to-end service: assay design, sample processing, data interpretation
For research on infectious diseases, immunology, oncology, or therapeutic antibody development, the 100 glycan microarray platform developed at Creative Biolabs provides a powerful solution for high-throughput profiling of glycan-binding interactions with exceptional sensitivity, reproducibility, and scalability. To request a quote or project consultation, contact us today to discuss how the 100 glycan microarray can advance your project.
Published Data
Mapping Anti-Glycan Antibody Specificity
A team sought to validate the glycan-binding profile of a monoclonal antibody candidate targeting sialyl-Tn (STn) antigen1, a known tumor-associated carbohydrate antigen (TACA). Using 100 glycan microarray, the antibody displayed selective binding to α2,6-sialylated Core 1 O-glycans, while showing negligible affinity for non-sialylated or Core 2 structures. These results confirmed the antibody's specificity, supporting its progression into preclinical trials for breast and ovarian cancer immunotherapy.
Know more about Anti-Glycan Antibody Related Services
Reference:
- Eavarone, David A., et al. "Humanized anti-Sialyl-Tn antibodies for the treatment of ovarian carcinoma." PLoS One 13.7 (2018): e0201314. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1371/journal.pone.0201314
