Fc Glycosylation Analysis Service
Why Fc Glycosylation Matters?
The glycosylation pattern of the Fc region determines antibody functionality because it affects mechanisms including ADCC, complement activation, and Fcγ receptor interactions. Minor modifications in fucosylation, galactosylation, or sialylation at the Fc region significantly impact therapeutic antibody function and immune responses. Physiological and pathological states such as autoimmunity, infection, and pregnancy can be monitored through glycan modifications which act as sensitive biomarkers. Fc N-glycans typically consist of a biantennary core with variable decorations:
- Core fucose reduces binding to FcγRIIIa, dampens ADCC
- Galactose influences complement activation (via C1q binding)
- Sialic acid drives anti-inflammatory responses via Type II FcγRs
- Bisecting GlcNAc alters glycan conformation and immune modulation
- High-mannose forms are associated with increased clearance from serum
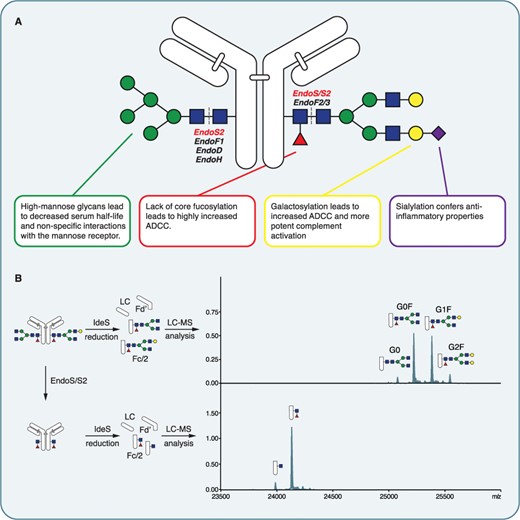 Fig.1 IgG Fc glycosylation.1
Fig.1 IgG Fc glycosylation.1
At Creative Biolabs, we offer a suite of specialized services to support Fc glycosylation research and development. Our antibody glycosylation analysis services include platforms tailored for monoclonal, polyclonal, Fab, and Fc-specific glycan profiling, leveraging cutting-edge mass spectrometry, enzymatic digestion, and labeling techniques. Whether you're engineering antibodies or investigating immune regulation, our Fc glycosylation analysis service provides precise, site-specific glycan characterization to accelerate your project with high accuracy and scientific depth.
Our Fc Glycosylation Analysis Workflow
Facing with various analytical challenges, Fc glycosylation analysis is technically demanding due to:
- Microheterogeneity: Over 30 glycoforms can co-exist in serum IgG.
- Site-specificity: Need to distinguish Fc from Fab glycans.
- Subtle changes: Small differences (e.g., partial afucosylation) have large functional consequences.
Thus, specialized analytical workflows are essential for accurate glycosylation mapping. At Creative Biolabs, we offer a comprehensive Fc glycosylation analysis platform optimized for sensitivity, resolution, and throughput. The process integrates:
1. Sample Preparation
- Total IgG is purified from serum or monoclonal production.
- The Fc domain is separated post-IdeS digestion, isolating it from Fab-derived glycans.
2. N-Glycan Release and Labeling
- PNGase F is used to cleave N-glycans enzymatically.
- Labeling enhances sensitivity for fluorescence detection and MS ionization.
3. LC-FLD/MS or MALDI-TOF-MS
- HILIC-UPLC-FLD enables quantitation of labeled glycans.
- MALDI-TOF-MS provides rapid, high-resolution glycan profiling for both intact Fc and released glycans.
4. Advanced Data Interpretation
- Glycan quantification (% relative abundance)
- Trait calculation: fucosylation, sialylation, galactosylation, bisection, high-mannose
- Comparison with reference standards or across batches
Sample Requirements
| Sample Type | Minimum Volume | Submission Notes |
|---|---|---|
| Purified IgG | ≥ 20 µg |
|
| Human or Mouse Serum/Plasma | ≥ 5 µL | |
| Hybridoma Culture Supernatant | ≥ 100 µL | |
| Recombinant Monoclonal Antibodies | ≥ 20 µg |
Key Applications
- Glycoform profiling of therapeutic mAbs (e.g., rituximab, trastuzumab)
- Stability and lot-to-lot consistency analysis
- Biosimilar comparability
- Correlating Fc glycoforms with ADCC, CDC, and anti-inflammatory activity
- Fc sialylation and placental antibody transfer studies
- Monitoring Fc glycosylation changes in autoimmune diseases (RA, SLE)
- Pregnancy-associated modulation of Fc glycans
Advantages of Our Service
✅ Site-specific profiling (Fc vs. Fab)
✅ High-throughput capabilities (96 samples per day)
✅ Broad glycan coverage including sialylation linkage-specificity
✅ Interpretation by senior glycobiologists
Services You May Be Interested in
Custom Antibody Glycosylation Service
Creative Biolabs supplies sophisticated antibody glycosylation solutions to advance therapeutic development and analytical characterization. Through cell line modification and enzymatic or chemoenzymatic techniques our custom service allows precise engineering of N- or O-glycans which enhances ADCC, CDC, PK, or antigen binding.
Glycan Profiling Service
We deliver extensive N-/O-glycan characterization through LC-ESI-MS/MS and MALDI-TOF-MS with fluorescent labeling to generate detailed glycan trait information for quality control, biosimilar comparability studies and biomarker research.
Glycosylation Site Mapping Service
Our glycosylation site mapping service provides advanced resolution by pinpointing glycan modifications to specific residues through LC-MS/MS glycopeptide analysis which enables evaluation of site occupancy and microheterogeneity as well as noncanonical glycosylation events.
FAQs
References:
- Sjögren, Jonathan, Rolf Lood, and Andreas Nägeli. "On enzymatic remodeling of IgG glycosylation; unique tools with broad applications." Glycobiology 30.4 (2020): 254-267. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1093/glycob/cwz085
- Bondt, Albert, et al. "Immunoglobulin G (IgG) Fab glycosylation analysis using a new mass spectrometric high-throughput profiling method reveals pregnancy-associated changes." Molecular & Cellular Proteomics 13.11 (2014): 3029-3039. https://doi.org/10.1074/mcp.M114.039537
- Wang, Taia T., and Jeffrey V. Ravetch. "Functional diversification of IgGs through Fc glycosylation." The Journal of clinical investigation 129.9 (2019): 3492-3498. https://doi.org/10.1172/JCI130029
- Zaytseva, Olga O., et al. "Fc-linked IgG N-glycosylation in FcγR knock-out mice." Frontiers in cell and developmental biology 8 (2020): 67. https://doi.org/10.3389/fcell.2020.00067