Engineering High-Affinity scFv for Next-Generation GD2-CAR-T Therapy
The fight against high-risk neuroblastoma, one of the most challenging and devastating solid tumors in children, has entered a new and promising era. For patients with relapsed or refractory disease, where traditional treatments offer grim prognoses, cellular immunotherapies are emerging as a powerful beacon of hope. Central to this revolution is the disialoganglioside GD2, a tumor-associated antigen that has become a validated and critical target for novel therapeutic strategies. The success of monoclonal GD2 antibody therapies, which have become a component of standard care, has paved the way for an even more potent approach: Chimeric Antigen Receptor (CAR) T-cell therapy. By engineering a patient's own T cells to recognize and attack cancer cells expressing the GD2 antigen, GD2-CAR-T therapy represents a living drug with the potential for profound and lasting responses. This technology has rapidly evolved through distinct designs, from simple first-generation constructs to more complex second- and third-generation CARs that incorporate additional co-stimulatory domains to enhance T-cell activation and persistence, as depicted in Fig.1.
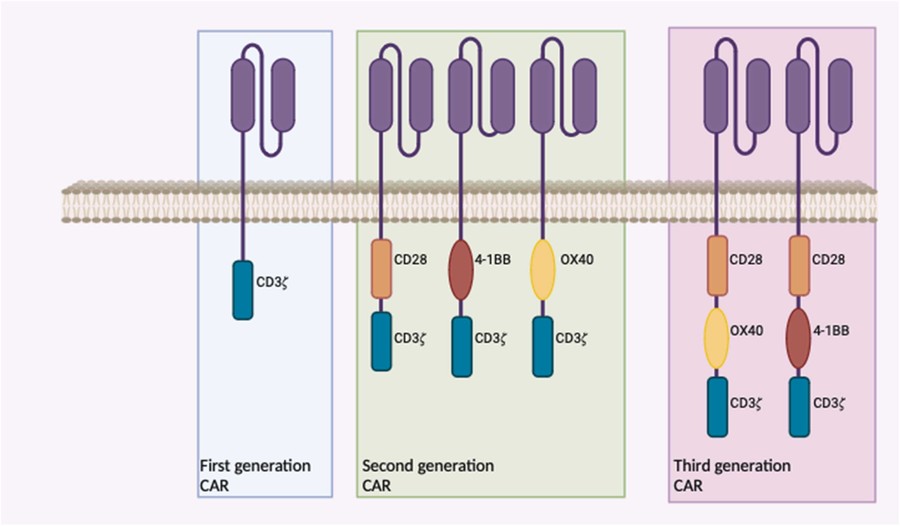 Fig.1 Evolution of chimeric antigen receptor (CAR) constructs for GD2-targeted therapy.1
Fig.1 Evolution of chimeric antigen receptor (CAR) constructs for GD2-targeted therapy.1
However, the journey to perfecting this therapy is complex. The efficacy and safety of a CAR-T cell are not determined by a single factor but by the sophisticated interplay of its components. At the very heart of the CAR construct lies the single-chain variable fragment (scFv)—the molecular guidance system that dictates target recognition. The binding characteristics of this scFv, particularly its affinity, are arguably the single most critical determinant of the CAR-T cell's ultimate success or failure. At Creative Biolabs, we understand this complexity, so we have summarized the critical bottlenecks in current GD2-CAR-T research, focusing on the challenges of affinity, on-target/off-tumor effects, and immunogenicity. And we will explore why an optimized, custom-developed scFv is paramount and how advanced antibody engineering services are paving the way for safer, more effective next-generation neuroblastoma therapy. Our expertise extends beyond the scFv to advise on the entire construct, including the optimal spacer and hinge domains needed for proper immune synapse formation. We partner with our clients to ensure the world-class scFv we develop is integrated into a CAR architecture designed for maximal impact.
The Double-Edged Sword: scFv Affinity in GD2-CAR-T Design
The scFv is an engineered protein fragment comprising the variable regions of the heavy (VH) and light (VL) chains of an antibody, joined by a flexible peptide linker. In a CAR construct, it is the scFv's job to identify and bind to the GD2 antigen on the tumor cell surface, thereby initiating the T-cell's cytotoxic response. The strength of this binding interaction is known as affinity. While it's intuitive to think that a stronger bond (higher affinity) would lead to a better outcome, the reality in CAR-T cell biology follows a more nuanced principle of optimal affinity: the affinity must be just right.
The Optimal Affinity Range for CAR Function
- Too Low: If the affinity is insufficient, the CAR-T cell may not form a stable immunological synapse with the tumor cell, failing to trigger a potent and sustained activation signal. This results in poor tumor clearance.
- Too High: Paradoxically, excessively high affinity can be detrimental. It can lead to severe side effects and can functionally impair the T cells themselves. For example, preclinical studies using a high-affinity variant of the common 14.18 anti-GD2 antibody (E101K) or the higher-affinity 3F8 antibody induced fatal neurotoxicity in some animal models.
- Just Right: An intermediate affinity level is often optimal. It provides a strong enough signal to activate the T cell effectively while allowing for serial detachment and re-engagement with multiple tumor cells. This balance promotes sustained killing and prevents premature T-cell exhaustion.
This delicate balance underscores the necessity of moving beyond off-the-shelf binders and toward precision-engineered scFvs for every new CAR-T program.
Navigating the Bottlenecks in GD2-CAR-T Development
Most first-generation GD2-CAR-T constructs have utilized an scFv derived from the murine monoclonal antibody 14.18 (also known as 14G2a). While foundational, this binder and others have revealed several key challenges that must be overcome to advance the field.
Challenge 1: Tonic Signaling and T-Cell Exhaustion
One of the most significant risks associated with high-affinity scFvs is tonic signaling. This is a phenomenon where the CAR molecules on the T-cell surface signal in the absence of the target antigen, for example, by clustering together during the manufacturing process. This chronic, low-level stimulation can drive the T cells into a state of immunological exhaustion, a state of hypo-responsiveness that severely limits their anti-tumor functionality once infused into the patient. Preclinical studies have demonstrated that a modified high-affinity version of the 14.18 scFv can cause severe tonic signaling, resulting in functionally impaired CAR-T cell products. While it remains an area of active investigation—with some studies failing to demonstrate exhaustion with the unmodified 14.18 scFv —the risk is significant enough that controlling tonic signaling through rational scFv design is a top priority.
Challenge 2: On-Target, Off-Tumor Toxicity
A crucial safety concern for any CAR-T therapy is "on-target, off-tumor" toxicity, which occurs when the CAR-T cells attack healthy tissues that express the target antigen at low levels. The ganglioside GD2 antibody target is a prime example of this challenge. GD2 is expressed not only on neuroblastoma cells but also on normal tissues, including peripheral nerves and cells within the central nervous system (CNS). This raises concerns about inducing neurotoxicity, a side effect observed with high-dose monoclonal antibody infusions. Reassuringly, clinical trials of GD2-CAR-T cells have not reported cases of severe neurotoxicity that could be unequivocally attributed to on-target, off-tumor reactivity. Neurological symptoms that have occurred are often considered manifestations of Cytokine Release Syndrome (CRS). Nonetheless, the potential for toxicity remains, especially as therapies become more potent. Engineering an scFv with an optimized off-rate (the speed at which it detaches from its target) could be a key strategy to mitigate this risk, allowing the CAR-T cells to effectively kill high-GD2-expressing tumor cells while sparing normal tissues with low-level expression.
Challenge 3: Immunogenicity
Many neuroblastoma patients entering CAR-T trials have previously been treated with therapeutic murine antibodies like dinutuximab. This prior exposure can lead to the development of human anti-mouse antibodies (HAMAs). If the CAR-T cell uses an scFv of murine origin, these pre-existing antibodies could potentially neutralize the CAR-T cells, compromising their expansion and persistence. While this concern remains largely theoretical and has not yet been definitively observed to limit CAR-T engraftment in clinical studies, it represents a logical vulnerability. The most effective way to de-risk this potential issue is to develop fully human or humanized scFvs, such as the huk666 binder evaluated in clinical studies by the UCL group.
Our Solution: Precision scFv Engineering for a Superior CAR
Overcoming these multifaceted challenges requires a move away from simply adopting existing antibody fragments and toward a paradigm of bespoke, purpose-built scFv development. At Creative Biolabs, we specialize in providing this level of precision through a suite of state-of-the-art antibody engineering technologies. Our services are designed to deliver an scFv that is a finely tuned component optimized for your specific CAR construct.
Advanced Phage Display Screening
The journey begins with discovery. Instead of being limited to the handful of publicly known binders, our clients gain access to our unparalleled phage display libraries. These vast collections of antibody fragments (including large-capacity human and synthetic libraries) serve as the ideal starting point for identifying completely novel scFvs against the GD2 antigen. This allows for the selection of binders with unique epitopes and inherent biophysical properties that may be more suitable for CAR-T applications than traditional options.
Expert Affinity Maturation
Discovery is just the beginning. Our true expertise lies in our ability to perform meticulous affinity maturation. This process allows us to take a promising scFv and rationally engineer its binding kinetics. We can fine-tune the association rate (kon), dissociation rate (koff), and overall affinity (KD) to hit the optimal kinetics for therapeutic efficacy. For GD2-CAR-T, this could mean engineering an scFv with a moderately high affinity but a faster off-rate. This profile could be sufficient to trigger potent T-cell killing of tumor cells while minimizing the prolonged signaling that leads to tonic signaling and reducing the risk of binding too tightly to low-density GD2 on normal tissues.
Comprehensive Humanization Services
To directly address the challenge of immunogenicity, we provide expert antibody humanization services. Our team can seamlessly convert a promising murine scFv into a humanized version, grafting the critical antigen-binding loops (CDRs) onto a human framework. This process, which led to the development of binders like huk666, significantly reduces the risk of rejection by the patient's immune system, paving the way for better CAR-T cell persistence.
Accelerate Your Research with Creative Biolabs
Creative Biolabs is committed to empowering researchers at the forefront of cancer immunotherapy. We offer a dual-pronged approach to support your GD2-targeting research programs.
Custom Anti-GD2 Antibody Development Service
For those developing novel CAR-T, CAR-NK, or other immunotherapies, our end-to-end custom antibody development service is the definitive solution. We provide a seamless workflow from initial target analysis and library screening to affinity maturation, humanization, and production of a final, CAR-T-ready scFv tailored to your precise specifications. Partner with us to engineer a truly next-generation binder for your therapeutic candidate.
Ready-to-Use Anti-GD2 Antibody Products
For foundational research, target validation, assay development, and benchmarking, we also offer a comprehensive portfolio of high-quality, off-the-shelf anti-GD2 antibody products. These rigorously validated reagents provide the reliability and consistency your research demands.
GD2-CAR-T therapy holds immense promise for changing the treatment landscape of neuroblastoma. Recent clinical data has confirmed that targeting GD2 is a tractable and effective strategy, capable of inducing significant and sustained clinical responses. However, realizing the full potential of this approach requires us to look beyond first-generation designs and confront the challenges of T-cell exhaustion, off-tumor toxicity, and immunogenicity head-on. The path forward lies in the rational and precise engineering of the CAR's core component: the scFv. By moving toward custom-developed binders with optimized affinity and humanized frameworks, we can build safer, more persistent, and more powerful cellular therapies. Creative Biolabs stands ready to be your expert partner in this mission. Contact our specialists today to discover how our advanced antibody engineering platforms can help you design and develop the next breakthrough in neuroblastoma therapy.
Reference:
- Anderson, John, Giuseppe Barone, and Alexandra Zehner. "GD2 targeting CAR T cells for neuroblastoma." EJC Paediatric Oncology 4 (2024): 100179. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1016/j.ejcped.2024.100179
Supports
- Glycolipid
- GM3 Antibody in Cancer Immunotherapy
- GM3 Ganglioside in Disease & Anti-GM3 Antibody Tools
