Custom Glycosylation of Peptides
Peptide-based therapeutics have surged in popularity due to their high specificity, low toxicity, and versatile design potential. However, native peptides often suffer from poor pharmacokinetics, rapid enzymatic degradation, and immunogenicity. At Creative Biolabs, we provide comprehensive custom glycosylation services for peptides, spanning from synthesis to analytical characterization, designed to meet the diverse and demanding research needs.
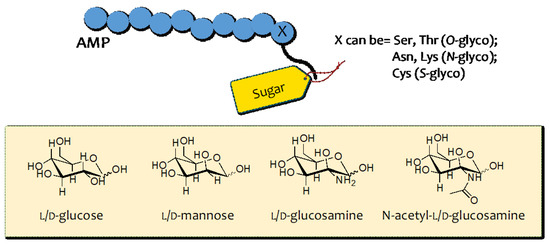 Fig.1 Glycosylation strategies for linking sugar moieties to antimicrobial peptides (AMPs).1
Fig.1 Glycosylation strategies for linking sugar moieties to antimicrobial peptides (AMPs).1
Why Glycosylate Peptides?
By adding glycans to peptides, we can significantly increase their solubility and bioavailability, while also extending their serum half-life by masking proteolytic cleavage sites. Glycosylation can facilitate proper folding and reduce peptide aggregation, ensuring better stability. Furthermore, it can influence receptor binding, enhance immune evasion, and direct peptides to specific cellular compartments, such as lysosomes or exosomes, for targeted delivery. These modifications are essential for improving peptide performance in research.
Peptide Glycosylation We Offer
At Creative Biolabs, we offer a wide array of peptide glycosylation options tailored to suit your research needs. The types of glycosylation we specialize in include:
- N-linked Glycosylation: This modification occurs at asparagine residues, and our service ensures precise incorporation of glycan structures at these sites, optimizing peptide functionality for therapeutic applications.
- O-linked Glycosylation: O-linked glycosylation is the process of attaching glycans to serine or threonine residues. This modification is often used to enhance mucin-like properties or modulate peptide interactions with specific receptors.
Synthetic Strategies for Glycopeptides
Custom glycopeptide synthesis requires precise control over both peptide sequence and glycan structure. We support multiple platforms:
Solid Phase Peptide Synthesis (SPPS) with Glycosylated Amino Acids
SPPS using pre-glycosylated amino acids allows site-specific control over glycosylation. Our solutions include:
- Triazole-linked glycosyl amino acids for click chemistry assembly.
- Fully protected glycosylated serine/threonine/asparagine derivatives.
- Custom synthesis of rare or tailored glycoforms.
Liquid Phase-based Glycopeptide Synthesis
While solid-phase synthesis is widely used for short glycopeptides, liquid-phase synthesis offers unique advantages for producing longer or more complex glycopeptides, especially when high stereochemical control and scalability are required. This approach is particularly effective for:
- Synthesizing N-glycosylated peptides with large, branched glycans.
- Incorporating chemically sensitive glycans that may degrade under solid-phase conditions.
- Generating libraries of glycopeptide analogs for structure–activity relationship studies.
Post-Synthetic Glycan Conjugation
Using chemoselective handles introduced during peptide synthesis, glycans can be attached post-synthetically:
- S-alkylation of cysteine residues for stable β-glycosidic analogs.
- Azide–alkyne cycloaddition to introduce triazole-linked sugars.
- N-terminal modifications using aldehyde or oxime chemistry.
Discuss Your Project
Analytical Tools for Glycosylated Peptides
Accurate glycopeptide characterization is necessary. Our comprehensive analytical suite includes innovative techniques for detailed glycopeptide characterization. We use LC-MS/MS to offer reliable sequence confirmation and site-specific glycosylation profiling. MALDI-TOF MS is used to determine the precise mass of glycopeptides, whereas HPLC (RP and HILIC modes) assures high purity and effective isomer separation. Lectin blotting is a very useful method for finding specific glycan patterns. Furthermore, ionization investigations using ESI and MALDI provide insights into quantifying ionization differences between glycosylated and unmodified peptides, allowing for a better understanding of their biological features. We also provide specialized peptide glycosylation testing, such as experiments that compare glycosylated and unmodified peptide behavior under enzymatic, thermal, and immunological conditions.
Our Custom Peptide Glycosylation Workflow
- Peptide Design & Sequence Selection
- Site Selection for Glycosylation
- Chemical or Enzymatic Synthesis
- Conjugation with Defined Glycans
- Purification and Characterization
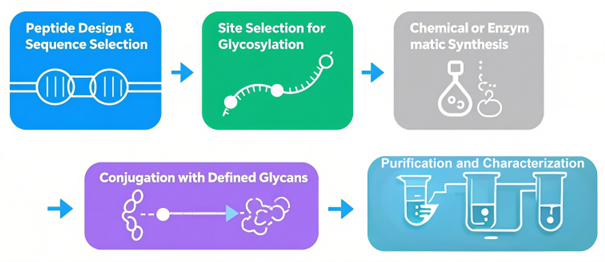 Fig.2 Custom peptide glycosylation workflow.
Fig.2 Custom peptide glycosylation workflow.
Related Custom Glycosylation Services
At Creative Biolabs, we offer a broad range of custom glycosylation services to support your research across multiple biomolecule types. Our comprehensive suite of glycosylation solutions is designed to enhance the properties and functionality of your research peptides, proteins, and other biomolecules. These services include:
-
Custom Glycosylation of Antibodies
We specialize in glycoengineering antibodies to optimize their effector functions, half-life, and receptor binding. -
Custom Glycosylation of Proteins
We provide precise glycosylation of recombinant proteins to optimize their stability, function, and therapeutic potential. -
Custom Glycosylation of Cell Membranes
We offer custom glycosylation services for membrane proteins to enhance their targeting, stability, and interaction with other cellular components. -
Custom Glycosylation of Bacterial Membranes
Tailored glycosylation of bacterial membrane proteins to improve their functionality in vaccine development. -
Custom Glycosylation of Small Molecules & Nucleic Acids
We also provide glycosylation solutions for small molecules and nucleic acids, enabling targeted drug delivery and improved bioactivity. -
Custom Glycosylation of Lipids
Enhancing lipid-based molecules with specific glycan modifications for applications in drug delivery and molecular recognition.
Peptide glycosylation is no longer merely a structural curiosity; it is a valuable strategy for influencing therapeutic behavior. Whether you want to improve delivery, tune immunological interactions, or improve peptide pharmacokinetics, Creative Biolabs has the knowledge, resources, and accuracy to bring your glycopeptide projects to life. Each service is backed by cutting-edge facilities, technical experience, and a dedication to delivering the best results. Contact us today to discuss your custom peptide glycosylation project or request a quote.
FAQs
How does glycosylation affect the activity of peptide hormones?
Glycosylation enhances peptide hormone stability by increasing serum half-life and preventing enzymatic degradation. It also influences receptor binding, improving specificity and enhancing signaling. This modification can improve bioactivity by promoting optimal conformation for receptor interaction. However, improper or excessive glycosylation may disrupt function, altering receptor recognition. Creative Biolabs offers detailed analysis services to assess how glycosylation influences your peptide hormone's activity and optimize its potential.
Why doesn't the amino terminal signal peptide get cut during glycosylation?
Signal peptide cleavage may not occur if glycosylation interferes with the recognition by signal peptidase or if the peptide structure hinders cleavage. In some cases, the signal peptide remains attached to the mature peptide, especially in glycoproteins requiring stable membrane insertion. Sequence motifs and structural factors can also prevent proper cleavage. At Creative Biolabs, we provide insights into peptide processing and glycosylation to help you optimize your peptide design for specific functions.
Reference:
- Bellavita, Rosa, et al. "Glycosylation and lipidation strategies: Approaches for improving antimicrobial peptide efficacy." Pharmaceuticals 16.3 (2023): 439. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/ph16030439
