Glycan Science: An Expert Review of Glycosylation, Key Technologies, and Research Frontiers
Introduction: What is Glycan Science?
As our understanding of cellular biology grows deeper, we've started to recognize a really profound and complex layer of information that exists beyond proteins themselves. This is the realm of glycobiology—the study of glycans' structure, how they're made, and what they do. Glycans are complex carbohydrate structures that cover cells. Unlike proteins, they aren't directly coded by a template; instead, enzymes work together to synthesize them. The process of attaching these glycans to proteins and lipids is called glycosylation, and it's one of the most common and intricate types of post-translational modification. Just look at the Fig.1: it shows various glycan structures—like O-glycans such as Sialyl core 1 O-glycan, T antigen O-glycan, Tn antigen O-glycan, and N-glycans like Oligomannose N-glycan, bisected complex-type N-glycan—attached to proteins on the cell membrane and how they're involved in signaling. When a protein has glycans attached to it, it becomes a glycoprotein, a hybrid molecule where those carbohydrate attachments permanently change its identity and function. The significance of glycans is huge in nearly every biological process. They control cell-to-cell communication, influence how the immune system recognizes things, and serve as docking spots for pathogens. When glycosylation goes wrong, it can cause terrible diseases, ranging from congenital disorders to cancer. Understanding glycosylation is one of the major challenges in modern biology, and at Creative Biolabs, we're committed to providing the tools and knowledge to help researchers figure it out.
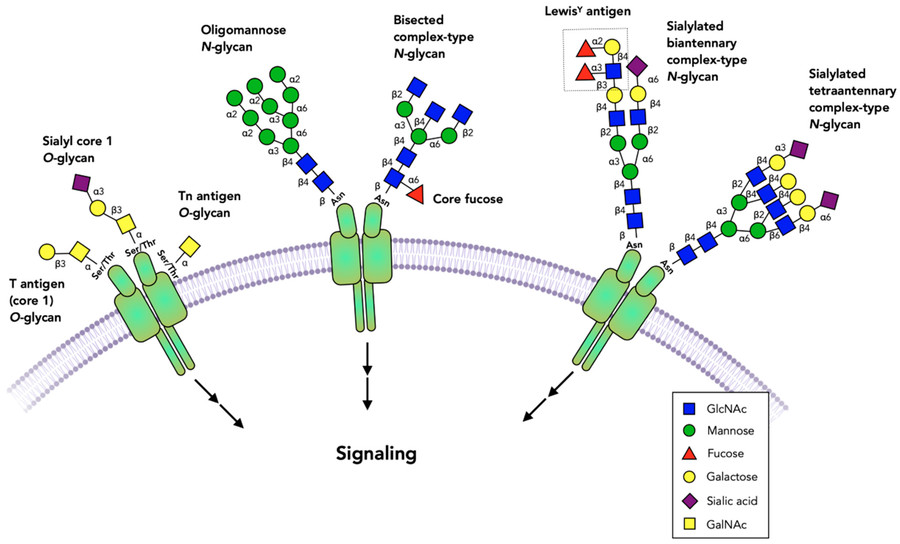 Fig.1 Common glycan structures and their roles in cell signaling.1
Fig.1 Common glycan structures and their roles in cell signaling.1
Functional Roles of Key Glycan Structures
The diversity of glycan structures is matched only by the diversity of their functions. Across different biological contexts, specific glycan motifs have been identified as pivotal players in health and disease.
Tumor Glycosylation in Cancer Progression
Nowhere is the impact of altered glycosylation more evident than in oncology. Tumor glycosylation is a hallmark of cancer, where malignant cells display a radically different sugar coating compared to their healthy counterparts. These aberrant structures, known as tumor-associated carbohydrate antigens (TACAs), are not passive bystanders. For example, the appearance of sialylated Lewis antigens (like Sialyl Lewis A and Sialyl Lewis X) on the cell surface promotes metastasis by allowing cancer cells to bind to selectins on blood vessel walls. Other TACAs, such as the Tn and Sialyl-Tn antigens, are associated with poor prognosis and help cancer cells evade the immune system. This makes them prime candidates for both glycan biomarkers and therapeutic targets.
Glycans in Immune Regulation and Response
The immune system is exquisitely sensitive to glycan structures. One of the most critical examples is antibody glycosylation. The Fc region of an IgG antibody contains a conserved N-glycan, and subtle changes to this glycan's structure can dramatically alter the antibody's function. For instance, removing fucose from this glycan can enhance its ability to trigger ADCC by over 100-fold—a principle now used to engineer more potent therapeutic antibodies. Beyond antibodies, glycan-binding proteins like Siglecs and Galectins act as immune checkpoints, recognizing specific glycans to either activate or suppress immune responses, making them exciting targets for immunotherapy.
Glycans in Pathogen Interactions and Neurobiology
In the constant battle between host and pathogen, glycans are on the front lines. Many viruses, including HIV and SARS-CoV-2, cloak their surface proteins in a dense "glycan shield" to hide from the host's immune system. Conversely, they often use host cell glycans as receptors to gain entry. Bacteria utilize surface polysaccharides for similar purposes, and these structures are the basis for many successful vaccines. In neuroscience, glycans are essential for proper neural development and function. Gangliosides, a class of glycans found on nerve cells, play roles in cell signaling and synaptic transmission. The abnormal accumulation or presentation of gangliosides like GM1 and GD2 is linked to neurodegenerative diseases and cancers like neuroblastoma.
Key Glycan Research Technologies
To study something as complex as the "glycome"—the entire complement of glycans in an organism—scientists require a sophisticated set of tools. The journey from a biological sample to meaningful data involves several critical steps, each with specialized technologies.
Release and Structural Analysis
The first step in any comprehensive glycan analysis is often to liberate the glycans from their parent proteins or lipids. This can be achieved through enzymatic methods, with enzymes like PNGase F being the gold standard for releasing N-linked glycans. Alternatively, chemical methods such as β-elimination are used for O-linked glycans. Once released, these glycans are separated using HPLC or UPLC, which sorts them based on size, charge, and structure.
Following separation, the real detective work begins. The undisputed workhorse for determining glycan structure is mass spectrometry. The field of mass spectrometry glycans has evolved rapidly, with techniques like MALDI-TOF providing rapid profiling and LC-MS/MS enabling detailed sequencing and linkage analysis. For an unambiguous, atom-by-atom view of a glycan's three-dimensional structure, NMR remains the ultimate authority, though it requires larger sample quantities and specialized expertise.
Functional and Cellular Studies
Knowing what a glycan is only part of the story; we also need to understand what it does. That's where functional tools come in. The glycan array is a powerful high-throughput platform—think of it as a microscope slide with hundreds of different, well-defined glycan structures dotted on it. When researchers expose this array to a protein, antibody, or even a whole virus, they can quickly find out which glycans it binds to, uncovering vital functional interactions. There's also a related tool called the lectin array, which works the other way around: it uses known glycan-binding proteins (lectins) to analyze the glycosylation patterns in a sample. To observe glycans in their natural environment—on a cell's surface or inside a tissue—we use cellular analysis methods. Take metabolic labeling, for example: cells are given sugar precursors with chemical tags, and these cells then incorporate the tags into their glycans. After that, we can use microscopy to visualize these tags. What's more, the creation of highly specific anti-glycan antibodies has changed the field completely. Now, researchers can use standard techniques like IHC and flow cytometry to find exactly where specific glycan structures are and how much of them there is in complex biological systems. Looking to the future, the growth of glycomics and spatial glycomics is set to give us a never-before-seen look at how glycans vary from one cell to another.
Translational Glycobiology: From Biomarkers to Therapeutics
The ultimate goal of glycan science is to translate fundamental knowledge into real-world solutions. The field is now delivering on this promise across diagnostics, therapeutics, and biotechnology.
Glycan Biomarkers and Diagnostics
Because altered glycosylation is a feature of many diseases, glycans are excellent candidates for biomarkers. The measurement of specific glycoforms of proteins can offer diagnostic and prognostic value. For example, the CA19-9 test, which detects the Sialyl Lewis A antigen, is a widely used glycan biomarker for pancreatic cancer. Similarly, a specific glycoform of alpha-fetoprotein (AFP-L3) is used as a more specific marker for hepatocellular carcinoma. The ongoing discovery of new glycan-based biomarkers promises to usher in an era of more sensitive and specific diagnostics for a wide range of conditions.
Glycan-Targeted Therapeutic Strategies
The therapeutic landscape is being reshaped by our understanding of glycobiology. Glyco-engineering is now a standard part of biologic drug development, particularly for optimizing monoclonal antibodies to enhance their efficacy and safety. More directly, researchers are now targeting glycans themselves. This includes:
- Anti-Glycan Antibodies: Monoclonal antibodies that directly recognize and target TACAs on cancer cells, like the anti-GD2 antibody used to treat neuroblastoma.
- Antibody-Drug Conjugates (ADCs): Using an anti-glycan antibody to deliver a potent cytotoxic drug specifically to cancer cells, minimizing collateral damage.
- CAR-T Therapy: Engineering a patient's T-cells to recognize and attack cells bearing specific tumor glycans.
Carbohydrate-Based Vaccines and Biomaterials
The principles of glycobiology are also central to modern vaccinology. Many of the most effective vaccines against bacteria like Haemophilus influenzae type b (Hib) and Streptococcus pneumoniae are carbohydrate-based, using bacterial polysaccharides to elicit a protective immune response. Finally, the unique physical properties of certain glycans have made them invaluable in biomaterials. Hyaluronic acid, a large glycosaminoglycan, is widely used in cosmetics, drug delivery, and as a lubricant for treating osteoarthritis due to its remarkable ability to retain water and provide structural support.
At Creative Biolabs, we're proud to stand at the forefront of this dynamic field. We offer a full range of cutting-edge services and products—from custom glycan array screening to advanced glycan analysis—all designed to equip researchers with the tools they need to unlock the glycome's secrets. The future of medicine holds immense promise in this space, and we're right here to support you every step of the way.
Reference:
- Gao, Yin, et al. "Role of glycans on key cell surface receptors that regulate cell proliferation and cell death." Cells 10.5 (2021): 1252. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/cells10051252
Supports
- Understanding Glycosylation
- Glycosylation Influences Blood Type
- O-Linked Glycan
- N-Linked Glycan
- Glycolipid
- Lewis Antigen
- Blood Group Antigen
- Glycosaminoglycan
- Plant Glycan
- Bacterial Polysaccharide
- Polysaccharide-other
- Polysialic Acid
- Sulfo-Gal
- LacNAc
- Alpha-Galactosyl
- Mucin
- GM3 Antibody in Cancer Immunotherapy
- GM3 Ganglioside in Disease & Anti-GM3 Antibody Tools
- Engineering High-Affinity scFv for Next-Generation GD2-CAR-T Therapy
- Different Microarrays for Exploring Bacterial Surface Glycans
- A Four-Platform Guide to Glycosylation Microarrays
- Glycosylation Influences Blood Type
- GM3 Antibody in Cancer Immunotherapy
- GM3 Ganglioside in Disease & Anti-GM3 Antibody Tools
- Engineering High-Affinity scFv for Next-Generation GD2-CAR-T Therapy
- A Simple Guide to CANOMAD Syndrome
- Anti-Glycolipid Antibody Overview
- GM3 Ganglioside Overview
- Sulfatide and Anti-Sulfatide Antibodies Overview
- Globoside (Gb4) as a B19V Antiviral Target
- TACAs Overview
- Guide to Blood Group Antigens
- Comparing sLeA and sLeX Roles in Cancer
- CA19-9 as a Pancreatic Cancer Biomarker
- Lewis Antigen System Overview
- TACA-Targeted ADCs, CAR-Ts, and RICs
- Targeting Glycosylated MUC1
- Key TACAs: Tn, STn & TF Antigens
- Rhamnose & AuNP-MUC1 Strategy
- Targeting Glycosphingolipids: GD2, Globo H & GM3
- Fluorinated MUC1 Vaccines
- GAGs Overview
- Heparan Sulfate vs. Heparin
- CSPGs in Neural Regeneration
- Hyaluronic Acid in TME
- GAGs Sulfation
- Dermatan Sulfate & Wound Healing
- Keratan Sulfate Function
- Viral Glycan Shield
- HIV-1 Envelope Glycans
- Influenza HA & NA Glycosylation
- SARS-CoV-2 Spike Glycosylation
- Ebola & Marburg Glycan Shields
- Flavivirus E Protein Glycosylation
- HCV Envelope Glycans
- Herpesvirus Glycoproteins
- RSV G Protein Glycosylation
- Bacterial Cell Wall Glycans
- Lipopolysaccharide (LPS)
- Lipoteichoic Acid (LTA)
