Monoclonal Antibody Glycosylation Analysis Service
Glycosylation is a critical post-translational modification that shapes the behavior of monoclonal antibodies (mAbs), influencing everything from their 3D structure and biological activity to how they're processed in the body and their potential to trigger immune responses. At Creative Biolabs, we understand that nailing down the nuances of mAb glycosylation isn't just about science—it's about ensuring the success of your therapeutic candidates. Whether you're in early-stage research or scaling up for clinical trials, our glycosylation analysis of antibodies service is designed to deliver the insights you need, with a focus on accuracy, speed, and actionable data.
Structural Variations and Glycosylation Hotspots
mAbs are the workhorses of modern biotherapy, featuring a symmetrical Y-shaped structure with two heavy chains and two light chains. The antigen-binding Fab regions are all about specificity—locking onto targets with precision—while the Fc region plays a key role in activating the immune system. But here's the thing: their power lies in the details, and glycosylation is a major player in fine-tuning that power.
Where Glycosylation Happens
- Fc Region: The Asn297 site on the heavy chain's Fc region is the star player for N-linked glycosylation. This modification isn't optional; it's essential for maintaining the Fc's shape and enabling interactions with Fc receptors and complement proteins. Mess with this, and you could lose critical effector functions.
- Fab Region: While less common, glycosylation in the Fab region does happen—think cetuximab, where it impacts target binding. These sites are trickier to characterize, but our services include comprehensive profiling to catch even the subtle changes.
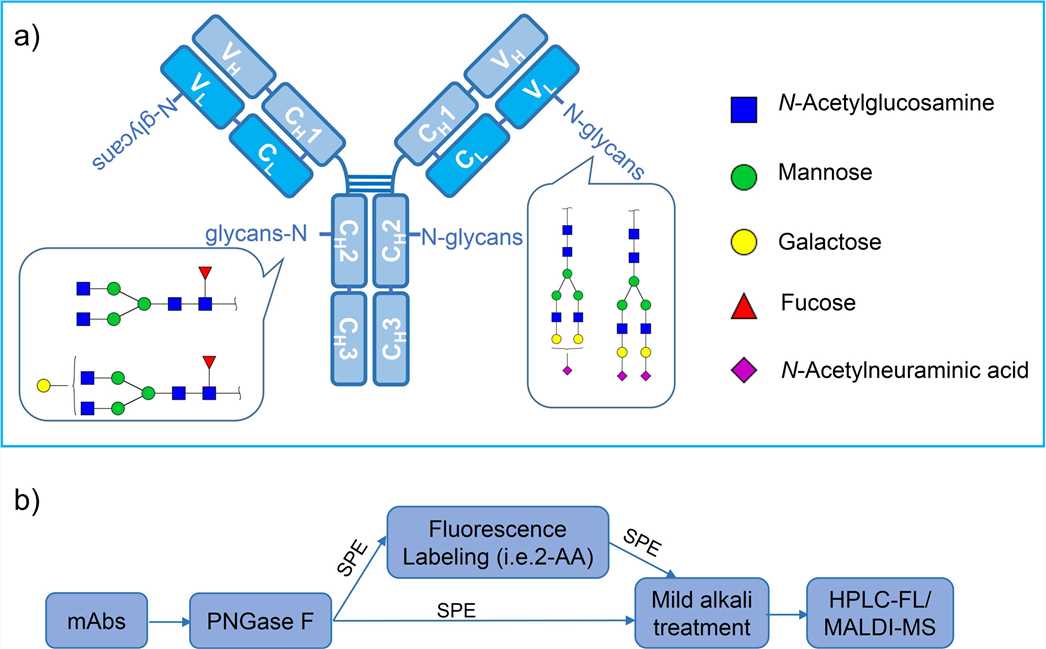 Fig.1 The structure of mAb with N-glycosylation sites.1,3
Fig.1 The structure of mAb with N-glycosylation sites.1,3
Glycan Structures Affect Functions
Fc glycans are mostly complex-type N-glycans, made up of building blocks like fucose, galactose, sialic acid, and bisecting GlcNAc. Variations like G0, G1, G2, or their sialylated/fucosylated forms are functional switches. For example, afucosylated glycans boost ADCC activity, making them heroes in cancer therapy. There are several potential impacts of Fc glycosylation on therapeutic mAbs as illustrated in the following chart (The symbol "↑" means positive impact; while "↓" represents negative impact).
| Glycans/glycosylation | Impacts |
|---|---|
| α1–3-galactose; N-glycolylneuraminic acid | ↓ binding to FcγRIIIa, ↓ ADCC; ↑ PK/PD |
| Terminal sialylation | ↓ binding to FcγRIIIa, ↓ ADCC, ↑ ADCP |
| Afucosylation | ↑ binding to C1q, ↑ CDC, moderate effect on ADCC |
| Galactosylation | ↑ PK/PD; ↑ binding to FcγRIIIa, ↑ ADCC; ↓ binding to C1q, ↓ CDC |
| High-mannose | ↑ PK/PD; ↑ binding to FcγRIIIa, ↑ ADCC; ↓ binding to C1q, ↓ CDC |
How Glycosylation Shapes mAb Performance
Effector Functions: Turn It Up or Down
- ADCC Amplification: Core fucose absence in Fc glycans cranks up binding to Fcγ receptors, supercharging antibody-dependent cellular cytotoxicity. Perfect for tumor-targeting mAbs where killing power matters.
- CDC Control: Terminal galactose and sialic acid levels dictate how well mAbs activate complement-dependent cytotoxicity. More galactose? Often means stronger CDC. It's all about balance.
Pharmacokinetics & Stability: Time in the Body Matters
Sialylation is like a protective cloak, reducing liver uptake and extending serum half-life. Meanwhile, glycan structure can shield mAbs from proteases, keeping them intact longer. Get this right, and you optimize dosing schedules and efficacy.
Immunogenicity: Avoiding the Body's Red Flags
Non-human glycans like α-Gal or Neu5Gc (common in murine or plant-based systems) can trigger unwanted immune responses. Our analytics help you spot these early, so you can engineer out risks and keep patients safe.
Know More about pAbs Glycosylation Analysis
Advanced Glycosylation Analysis Methods
Accurate analysis is the backbone of quality control and regulatory approval. Here's how we dig into the details:
HILIC for Separation
- It can separate glycans by polarity, often after fluorescent labeling to boost sensitivity.
- Its high resolution for distinguishing glycan variants—critical for spotting subtle changes between batches.
HPAEC-PAD with Simplicity
- There is no derivatization needed for this method. It can directly detect glycans by charge, speeding up analysis without extra steps.
- It is Ideal for quick screening or when you want to avoid labeling-induced variability.
LC-MS/MS for Structural Details
- We release glycans from mAbs, separate them by LC, then use MS to decode their structure—even isomers.
- We are able to provide you detailed maps of glycan composition, including branching and terminal modifications. Perfect for regulatory filings or complex R&D.
Our Custom Solutions
At Creative Biolabs, we specialize in tailoring antibody glycosylation analysis to meet your specific research and development needs. Whether you're conducting high-throughput screening or require ultra-sensitive detection for low-abundance samples, our team customizes methodologies to align with your objectives. We go beyond standard protocols by optimizing them, employing advanced tools such as PNGase F for precise glycan release and stable isotope labeling for accurate quantification.
Comprehensive Glycosylation Characterization
We offer a suite of services to thoroughly analyze antibody glycosylation:
- Glycan Composition Analysis: Identification and quantification of monosaccharide components (e.g., mannose, galactose, fucose, sialic acid) using techniques like acid hydrolysis or enzymatic release followed by HPAEC-PAD or mass spectrometry.
- Glycoform Heterogeneity Assessment: Evaluation of glycoform distributions (e.g., G0F, G1F, G2F, high-mannose) employing HILIC chromatography, MALDI-TOF MS, or UHPLC-FLD for separation and quantification.
- Glycosylation Site Mapping: Utilization of LC-MS/MS to pinpoint glycosylation sites, such as Asn297 in the IgG Fc region, and confirmation of glycan occupancy through peptide mapping.
Host Cell Selection
Your choice of host cell sets the stage for glycan profiles:
- CHO Cells: The go-to for therapeutic mAbs, mimicking human IgG but lacking α2-6 sialylation. We help you optimize culture conditions to hit your target profile.
- Murine Cells: Risk of non-human glycans? We'll flag them early, so you can adjust or engineer out issues.
- Yeast/Plant Systems: Unique glycans like β1-2 xylose? Our analysis ensures you understand immunogenic risks and how to mitigate them.
Applications
Early-Stage Design: Engineer for Success
Use our glycan profiling to guide cell line development. Want higher ADCC? We'll show you how afucosylation impacts Fcγ binding. Need better CDC? Let's map galactosylation patterns.
Process Optimization: Consistency is Key
We monitor glycosylation through upstream processes—media formulations, feeding strategies, even MnCl₂ supplementation—to ensure reproducibility.
Clinical Readiness: Regulatory-Ready Data
Our reports aren't just data dumps. They're comprehensive packages with raw data, statistical analysis, and clear insights to support IND filings. We know what regulators need, and we deliver it.
Personalized Medicine: Tailoring to Patients
As precision therapy grows, glycan profiles may predict patient response. Our advanced analytics help you explore these correlations, opening doors to stratified treatments.
Partnering with Creative Biolabs: Why We Stand Out?
At Creative Biolabs, our glycosylation analysis of monoclonal antibodies service is built on three pillars:
- Decades of experience in glycomics, from basic research to deeper analysis.
- Whether you need a standard panel or a custom workflow, we adapt to your timeline and goals.
- We don't just tell you what's there—we explain what it means for your mAb's efficacy, safety, and manufacturability.
Glycosylation is a critical quality attribute that can make or break your mAb. From effector function to immunogenicity, every glycan variant tells a story. At Creative Biolabs, we don't just analyze—we translate complex data into insights that drive better decisions, faster. Whether you're optimizing a lead candidate, troubleshooting a production issue, or preparing for clinic, our Glycosylation Analysis of Monoclonal Antibodies Services are your partner in precision. Let's talk about how we can tailor our expertise to your unique needs. Contact us today, and let's unlock the potential of your mAb's glycosylation profile together.
Published Data
Glycan heterogeneity of mAbs is of great importance in glycosylation profiling. Glycosylation is a critical quality attribute of mAb therapeutics, crucial for their safety and efficacy. The study employs hydrophilic interaction liquid chromatography-mass spectrometry (HILIC-MS) to characterize protein glycosylation. The following figure shows the released N-glycan map of the VRC01 monoclonal antibody analyzed by HILIC-UPLC-FLR-MS. A total of 53 glycans were identified and annotated, with 44 of them identified and annotated based on both GU values and accurate mass, while the remaining nine glycans were annotated based solely on GU values. The relative abundances of these glycans range from 0.1% to 11.92%. The figure also includes annotations for the retention times, observed GU values, observed masses, expected GU values, expected masses, predominant charge states, and response units of each glycan. These data provide a foundation for understanding the glycosylation profile of the VRC01 mAb.
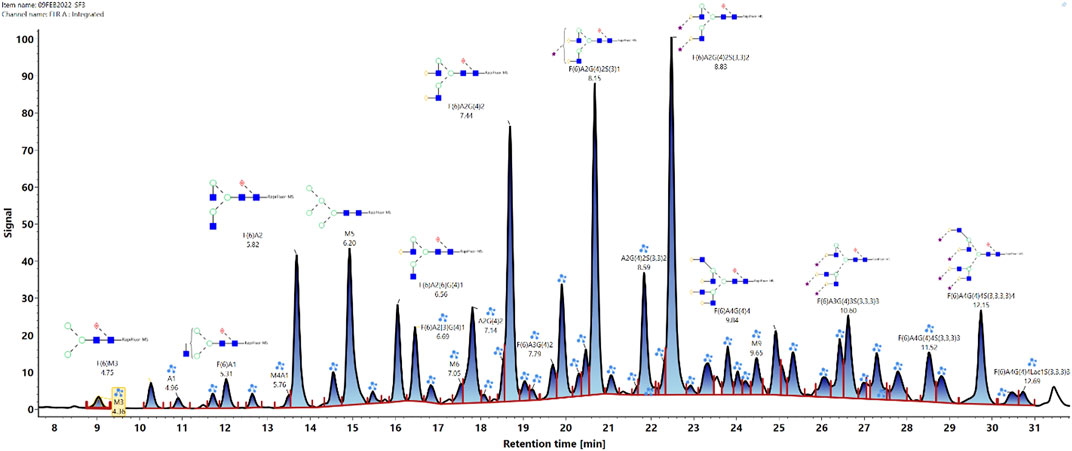 Fig.2 N-glycan profile of VRC01 monoclonal antibody by HILIC-UPLC-FLR-MS analysis.2,3
Fig.2 N-glycan profile of VRC01 monoclonal antibody by HILIC-UPLC-FLR-MS analysis.2,3
References:
- Liu, Sheng, et al. "Comprehensive N-glycan profiling of cetuximab biosimilar candidate by NP-HPLC and MALDI-MS." PLoS One 12.1 (2017): e0170013. https://doi.org/10.1371/journal.pone.0170013
- Singh, Sumit K., and Kelvin H. Lee. "Characterization of monoclonal antibody glycan heterogeneity using hydrophilic interaction liquid chromatography-mass spectrometry." Frontiers in Bioengineering and Biotechnology 9 (2022): 805788. https://doi.org/10.3389/fbioe.2021.805788
- Distributed under Open Access license CC BY 4.0, without modification.
- Senini, Isabella, et al. "Direct glycosylation analysis of intact monoclonal antibodies combining ESI MS of glycoforms and MALDI-in source decay MS of glycan fragments." Communications Chemistry 7.1 (2024): 203. https://doi.org/10.1038/s42004-024-01297-x