CA19-9 as a Pancreatic Cancer Biomarker: Limitations and New Frontiers
For decades, the fight against pancreatic cancer has been one of the most difficult challenges in modern oncology. Pancreatic ductal adenocarcinoma (PDAC), the most common form, is aggressive and invasive. Its high mortality rate is largely due to its silent progression, often leading to a diagnosis only at advanced stages when therapeutic options are limited. This late diagnosis has prompted an urgent, field-spanning search for more effective tools, particularly biomarkers—measurable indicators in the body that can signal the presence of disease. In this search, one name has dominated the conversation for over 40 years: CA19-9. But is CA19-9 the solution we've been looking for? As biological experts at Creative Biolabs, we've spent years on the front lines of this problem, partnering with researchers to build the high-precision tools needed to study it. This experience, particularly in our Custom Anti-sLeA (CA19-9) Antibody Development service, has given us a clear perspective. While CA19-9 is a valuable tool, it is not a straightforward "yes or no" test for pancreatic cancer. Its limitations are just as important as its uses.
This article, intended for research purposes only, provides an expert review of the CA19-9 biomarker. We will cover:
- What CA19-9 actually is (biologically).
- The critical, well-documented limitations that prevent its use for early diagnosis.
- The new frontiers of research that are redefining CA19-9, not just as a marker, but as a therapeutic target.
Understanding these details is the first step toward developing the next generation of precision tools necessary to make significant progress against this devastating disease.
What Is CA19-9 (Sialyl-Lewis A)?
CA19-9, also called sialyl-Lewis A or sLeA, is a glycan with complex carbohydrate structures. In pancreatic cancer cells, the production of sialyl-Lewis A is dramatically increased. The cancer cells not only display it on their surface but also shed it in large amounts into the bloodstream. This sLeA is what we detect in a blood test as "elevated CA19-9." Because tumors so heavily shed it, CA19-9 is currently the only FDA-approved serum biomarker widely used for pancreatic cancer management, with clinical application limited to monitoring treatment response and detecting recurrence. Due to significant limitations, CA19-9 is not used for screening the general population or for the initial diagnosis of cancer. Its clinical application is confined to:
- Monitoring treatment response in patients already diagnosed.
- Watching for disease recurrence after surgery.
The Core Challenge: Critical Limitations of CA19-9
Why isn't CA19-9 the golden ticket for early diagnosis? The answer lies in two major biological hurdles: false negatives and false positives.
Limitation 1: The "Lewis-Negative" Patient Challenge (False Negatives)
The biggest limitation of CA19-9 has nothing to do with the cancer; it is related to the patient's genetics. The synthesis of the sialyl-Lewis A glycan is a multi-step process. The final step requires a specific enzyme, a fucosyltransferase (FUT3), which is encoded by the "Lewis gene". However, approximately 5% to 10% of the population has a "Lewis-negative" phenotype. This means they have a genetic variation that results in a non-functional Lewis enzyme. These individuals are biologically incapable of producing the sLeA structure. Even if they have advanced, late-stage pancreatic cancer, their bodies cannot make CA19-9. Their blood test will always come back negative or at zero. This creates an immediate and unavoidable "false negative" gap. Any research into new diagnostic assays or ELISA kit development based solely on CA19-9 will automatically fail for this entire segment of the population.
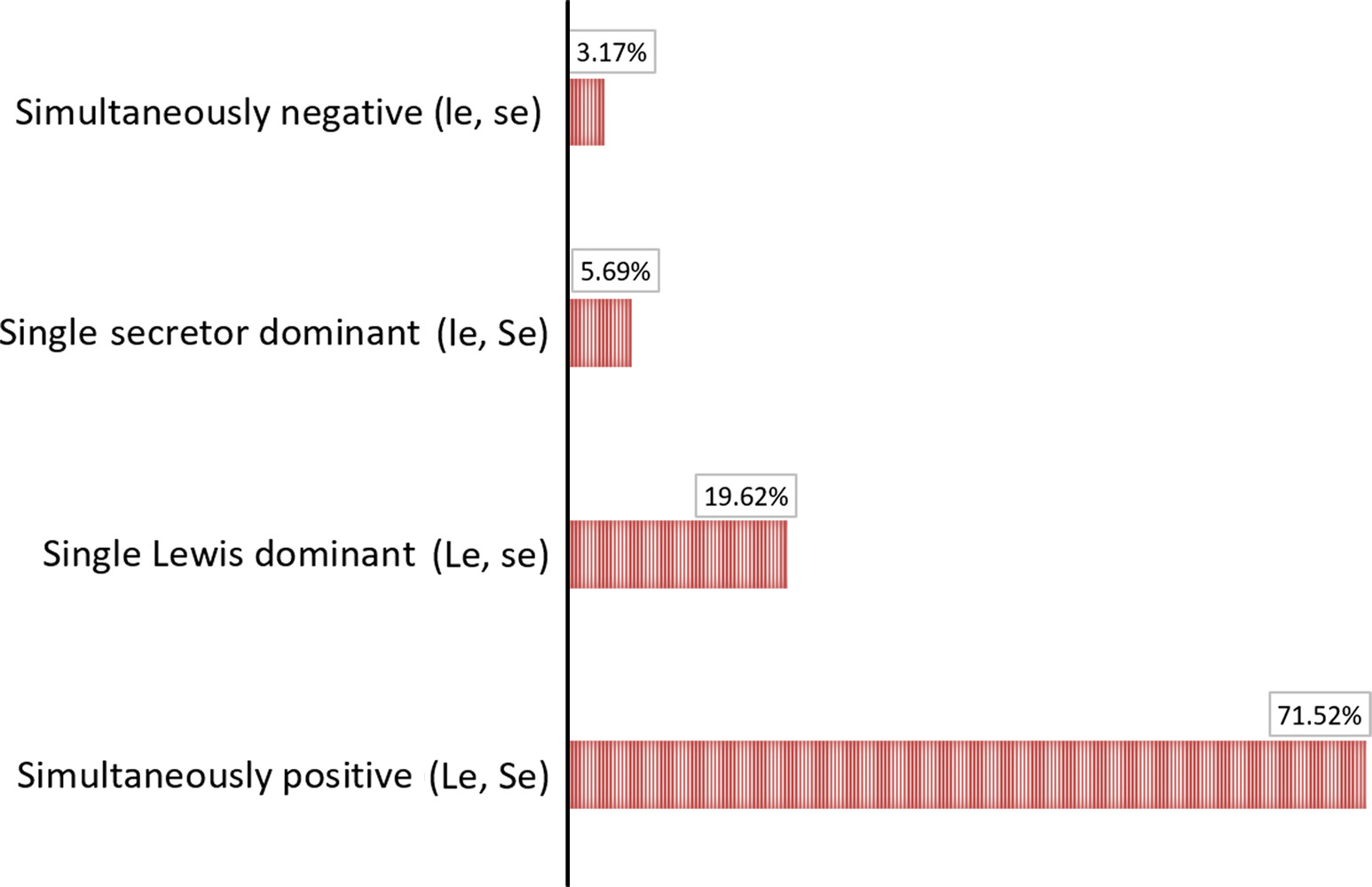 Fig.1 Serum CA19-9 levels across Lewis phenotypes in a Chinese cohort.1
Fig.1 Serum CA19-9 levels across Lewis phenotypes in a Chinese cohort.1
| Patient Phenotype | Lewis Gene (FUT3) Status | Can They Make sLeA (CA19-9)? | Test Result in Cancer |
|---|---|---|---|
| Lewis-Positive (90-95% of the population) | Active Enzyme | Yes | Positive (Elevated) |
| Lewis-Negative (5-10% of the population) | Inactive Enzyme | No | False Negative |
Limitation 2: The Specificity Problem (False Positives)
The second major issue is low specificity. A high CA19-9 level does not definitively mean pancreatic cancer.
Elevation in Other Cancers:
CA19-9 is not tumor-type-specific. Its elevation is also a common feature in many other malignancies, particularly other gastrointestinal cancer types. These include:
- Colorectal cancer
- Gastric (stomach) cancer
- Liver cancer
- Lung and breast cancer
Elevation in Benign Conditions:
More confusingly, CA19-9 levels can be very high in patients with non-cancerous conditions. The most common cause of a "false positive" is obstructive jaundice, which can be caused by benign gallstones or inflammation. This low specificity makes CA19-9 unreliable for diagnosis.
Limitation 3: Low Sensitivity in Early Stages
Finally, even in Lewis-positive patients who do have pancreatic cancer, the CA19-9 levels may not rise significantly during the earliest, most treatable stages of the disease. These three factors—the Lewis-negative gap, low specificity, and poor early-stage sensitivity—are why CA19-9 has failed as a screening tool, and why researchers are urgently seeking new frontiers.
The Evolving Role of CA19-9: From Marker to Target
Part 1: Moving Beyond a Single Marker
The limitations of CA19-9 have led the research community to a clear conclusion: a single biomarker is not enough. The future of early diagnosis lies in the development of biomarker panels. The goal is to combine CA19-9 with other molecular and circulating markers. By using an algorithm, a panel of multiple markers can provide a significantly more accurate "fingerprint" of the disease, thereby overcoming the limitations of any single one. Researchers are now testing CA19-9 in combination with many other promising circulating biomarkers.
- Carcinoembryonic Antigen (CEA): This is another well-known glycoprotein marker. On its own, CEA has even lower sensitivity and specificity for pancreatic cancer than CA19-9. However, some studies suggest that using CEA in conjunction with CA19-9 canhelp differentiate between benign and malignant pancreatic lesions.
- CA242: This is another carbohydrate antigen that is highly correlated with CA19-9 levels. Its complementary role is still under investigation, but it is a frequent candidate for new biomarker panels.
- Newer Circulating Markers: A new wave of research is focused on novel markers that show high promise. These include PIVKA-II (also known as DCP), GDF-15, and TIMP-1; however, these emerging markers require further validation compared to more established complementary markers, such as CEA and CA242. The hope is that a panel combining CA19-9 with one or more of these new markers could finally achieve the diagnostic accuracy needed for early detection.
Part 2: CA19-9 as a Biological Target
Perhaps the most exciting shift in modern pancreatic cancer research is this: What if CA19-9 isn't just a passive marker, but an active player in the disease? The evidence is overwhelming. The sialyl-Lewis A glycan is not merely shed waste by the tumor; it is also a key component of the tumor's structure. It is a functional tool that the cancer uses to grow and spread. This discovery has opened up a completely new frontier, redefining CA19-9 as a high-priority therapeutic target. Research shows that the sLeA glycan on the tumor cell surface actively:
- It is directly involved in accelerating cancer progression by glycosylating proteins.
- The sLeA glycan binds to E-selectin on endothelial cells, but also activates EGFR signaling pathways, representing a newly discovered mechanism driving pancreatic inflammation and cancer progression.
- It helps the tumor create its own new blood supply.
- It is involved in mediating the immunological response, helping the cancer hide from the body's immune system.
New Therapeutic Strategies Targeting CA19-9
This new understanding changes everything. If CA19-9 helps the cancer spread, then blocking CA19-9 could stop it. This has led to several new therapeutic strategies currently in preclinical or early-stage clinical development:
- Anti-CA19-9 Monoclonal Antibodies: Researchers are developing highly specific antibodies that bind directly to the sialyl-Lewis A structure on the surface of cancer cells. The goal is to use these antibodies to trigger the patient's own immune system to attack the tumor.
- Targeted Drug Delivery: The sLeA structure can be exploited as a specific molecular target for cancer-directed drug delivery. Scientists can attach potent chemotherapy drugs to nanoparticles designed to bind specifically to CA19-9, delivering the poison directly to the cancer cell while sparing healthy tissue.
- Biosynthesis Inhibition: Instead of targeting the sLeA structure itself, some strategies aim to shut down the factory. This involves developing drugs that block the enzymes responsible for building the CA19-9 molecule in the first place.
Why Better Custom Anti-sLeA (CA19-9) Antibodies Are Essential
These new frontiers—from highly sensitive, multi-plex diagnostic panels to cutting-edge sLeA-targeted therapies—all depend on one single, critical component: a better antibody.
The limitations of CA19-9 as a biomarker are, in many ways, the limitations of the antibodies used to detect it.
To explore this new frontier, researchers need custom-developed tools with exquisite specificity. You need:
- An antibody that binds only to sialyl-Lewis A with high affinity.
- An antibody that is guaranteed not to cross-react with similar-looking glycans (like sialyl-Lewis x, for example).
- An antibody that is robustly validated for modern, high-stakes applications like ELISA kit development, flow cytometry, and IHC.
- A reliable supply of this antibody that will not change from batch to batch.
This is where standard, off-the-shelf reagents often fail, and where specialized expertise becomes essential.
The Creative Biolabs Solution
At Creative Biolabs, we specialize in solving the most difficult antibody challenges. Targeting complex glycans, such as sialyl-Lewis A, is a core part of our expertise. Our custom anti-sLeA (CA19-9) antibody development service for pancreatic cancer research is designed from the ground up to provide the precision tools you need. We move beyond the limitations of older reagents by using modern antigen design, advanced discovery platforms, and rigorous glycoarray screening assays to deliver an antibody you can trust for your critical research.
CA19-9 remains a key molecule in pancreatic cancer research. Its well-documented limitations, particularly the Lewis-negative patient gap and low specificity, have rightly precluded its use as a single tool for early diagnosis. The future of diagnostics will depend on combining CA19-9 with other emerging biomarkers in complex panels. However, CA19-9's most promising future resides in its new role as a functioning biological target. Understanding its involvement in metastasis and immune evasion has elevated it from a basic marker to a prospective target for next-generation antibodies and cancer treatments. To lead this next wave of discovery, you'll need instruments that are more accurate and powerful than those used previously. Contact our team of glycan-antibody experts today, and let's build the custom tool your research deserves.
Reference:
- Guo, Meng, et al. "Distribution of Lewis and Secretor polymorphisms and corresponding CA 19‐9 antigen expression in a Chinese population." FEBS open bio 7.11 (2017): 1660-1671. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1002/2211-5463.12278
Supports
- TACAs Overview
- Guide to Blood Group Antigens
- Comparing sLeA and sLeX Roles in Cancer
- CA19-9 as a Pancreatic Cancer Biomarker
- Lewis Antigen System Overview
- TACA-Targeted ADCs, CAR-Ts, and RICs
