Lipopolysaccharide Analysis Service
What Is Lipopolysaccharide?
Lipopolysaccharide, commonly referred to as LPS or endotoxin, is a major component of the outer membrane of Gram-negative bacteria. Exclusive to Gram-negative bacteria, LPS must be rigorously detected and quantified in pharmaceutical manufacturing. This is critical for ensuring that biologics and parenteral drugs are free from endotoxin contamination. At Creative Biolabs, our specialized lipopolysaccharide analysis service enables researchers and drug developers to comprehensively characterize the structure, composition, and biological activities of LPS from diverse Gram-negative bacterial species. With several years of technical expertise and advanced analytical platforms, we provide end-to-end solutions for your lipopolysaccharide profiling needs.
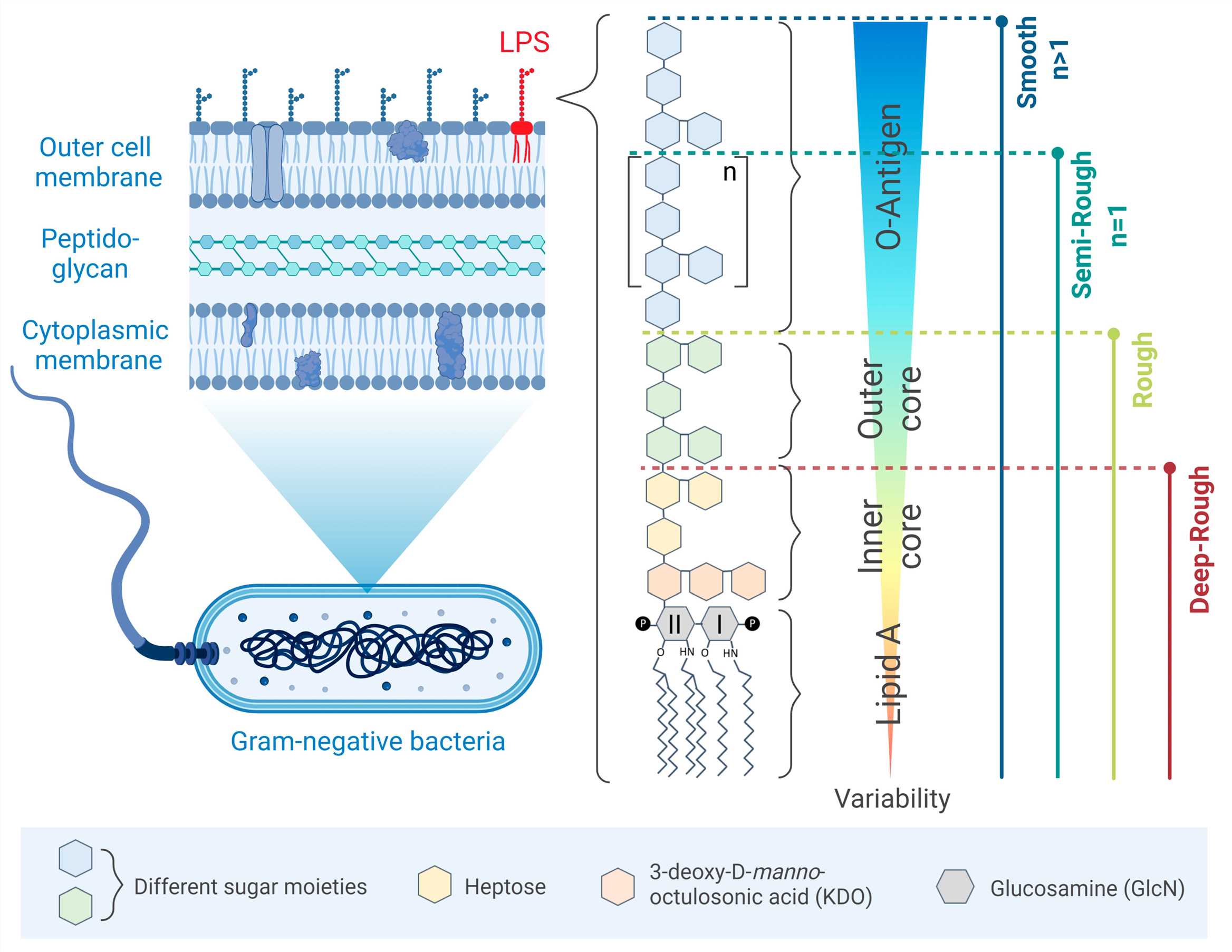 Fig.1 LPS structure.1
Fig.1 LPS structure.1
The Structure of Lipopolysaccharide
LPS consists of three major domains:
- Lipid A: This membrane-anchoring domain is responsible for most of the toxic activity associated with LPS. It consists of a phosphorylated glucosamine disaccharide backbone with multiple fatty acid chains.
- Core oligosaccharide: Bridging Lipid A and the O-antigen, this region contains unusual sugars such as heptoses and 3-deoxy-D-manno-octulosonic acid.
- O-antigen polysaccharide: A highly variable outermost region composed of repeating oligosaccharide units. It plays a key role in antigenicity and immune evasion.
Know more about Bacterial Polysaccharides
Why Lipopolysaccharide Analysis Matters
Lipopolysaccharide function extends well beyond structural support. As an essential virulence factor, LPS:
- Activates innate immune signaling via TLR4-MD2 complexes
- Triggers cytokine storms and septic shock in severe infections
- Contributes to antibiotic resistance by reinforcing outer membrane permeability barriers
For biopharmaceutical developers, controlling lipopolysaccharide endotoxin levels is not optional. LPS concentrations in injectable products are strictly limited by regulatory guidelines. Hence, precise characterization of lipopolysaccharides becomes vital in drug safety assessment. Moreover, for microbiologists, unraveling lipopolysaccharide microbiology opens doors to understanding host recognition mechanisms and microbial pathogenesis.
Creative Biolabs' Lipopolysaccharide Analysis Platform
Our analytical platform is designed to support multiple research and industrial applications. We combine advanced techniques to deliver accurate, reproducible, and customizable data packages for LPS characterization. To advance your research objectives, select the analysis type that aligns with your goals:
Structural Analysis for LPS
| Mass Spectrometry (MALDI-TOF, ESI-MS) | Elucidation of Lipid A structure, O-antigen length determination |
| NMR Spectroscopy | Atomic-level mapping of sugar moieties |
| HPLC | Separation of LPS subcomponents |
| SDS-PAGE with Silver Staining | Profiling ladder-like patterns of LPS species |
Endotoxin Quantification
| Limulus Amebocyte Lysate (LAL) Test | Gold standard for detecting lipopolysaccharide endotoxin in biopharmaceuticals |
| Recombinant Factor C (rFC) Assay | Animal-free method with high specificity for Gram-negative lipopolysaccharide |
| Monocyte Activation Test (MAT) | Human cell-based assay for functional LPS pyogenicity |
LPS Functional Evaluation
- TLR4 Activation Assays: Assessment of immune-stimulating properties of LPS
- Cytokine Profiling: Measurement of IL-6, TNF-α, and IL-1β production in response to LPS exposure
- Cellular Toxicity and Apoptosis Assays: Determination of cell viability post-LPS treatment
Talk to An Expert
Sample Requirements
To ensure accurate and reproducible results, Creative Biolabs recommends preparing samples according to the following specifications:
| Sample Type | Volume / Amount | Pay Attention to: |
|---|---|---|
| Bacterial Cell Pellet | ≥ 1 × 10⁹ cells | Avoid freeze-thaw cycles; wash with PBS before freezing |
| Purified Lipopolysaccharide | ≥ 50 µg | Provide extraction method and buffer composition |
| Culture Supernatant | ≥ 2 mL | Recommended: 0.22 µm filtration prior to shipment |
| Recombinant Protein Sample | ≥ 100 µg | Include expression system and purification method |
| Tissue Lysates (if applicable) | ≥ 200 µg total protein | Provide lysis buffer composition and origin (e.g., mouse spleen, etc.) |
If you're unsure about sample preparation, our technical team can provide pre-analysis consultation to tailor workflows for your specific needs.
Applications Across Fields
By integrating LPS and glycolipid profiling, Creative Biolabs supports comprehensive investigations into:
| Research Area | Role of Lipopolysaccharide Analysis |
|---|---|
| Glycans in bacterial and viral infections | Dissect the contribution of lipopolysaccharide in Gram-negative bacteria to immune evasion and adhesion |
| Vaccine development against Gram-negative pathogens | Characterize immunogenic O-antigen variants and endotoxin lipopolysaccharide activity |
| Microbiome and host immunity | Study LPS-triggered TLR4 pathways alongside microbiota-derived glycolipids |
| Therapeutic protein purity assessment | Detect lipopolysaccharide endotoxin contamination in biologics and cell culture reagents |
| Structural glycobiology | Correlate lipopolysaccharide structure with lipid-linked oligosaccharide architecture and function |
Why Partner with Creative Biolabs?
- PhD-led team's technical mastery in glycobiology and LPS research
- Each lipopolysaccharide sample is unique. We tailor protocols based on bacterial origin, purity level, and downstream application.
- Our team ensures that your data meets global regulatory requirements for endotoxin control.
Whether you need high-throughput endotoxin screening, in-depth structural mapping, or immunogenicity profiling, Creative Biolabs stands as your trusted partner. We also offer glycolipid analysis services to support research into glycans in bacterial and viral infections, helping elucidate host-pathogen interactions and guide vaccine and therapeutic development. Let our expertise in lipopolysaccharide analysis enhance the accuracy, safety, and regulatory compliance of your research. Contact us today to discuss your project or request a quote
FAQs
How do you test for lipopolysaccharides?
We use a multi-platform approach to detect and characterize lipopolysaccharides (LPS), also referred to as endotoxin lipopolysaccharide. The testing strategy depends on the purpose. At Creative Biolabs, lipopolysaccharides are tested using mass spectrometry for structural profiling, NMR for glycan composition, SDS-PAGE with silver staining for molecular weight patterns, and LAL or recombinant Factor C assays for quantifying endotoxin lipopolysaccharide levels.
How do you measure LPS function?
We assess LPS function using cell-based assays that measure Toll-like receptor 4 (TLR4) activation, cytokine production, and monocyte activation. Reporter assays quantify NF-κB signaling, while ELISA or multiplex platforms detect TNF-α, IL-6, and other cytokines, reflecting LPS-induced innate immune responses and pyrogenicity.
What is the function of the LPS?
Lipopolysaccharide stabilizes the outer membrane of Gram-negative bacteria, providing structural integrity and resistance to external stress. Its O-antigen facilitates immune evasion, while the lipid A domain interacts with host receptors. LPS also contributes to virulence, antibiotic resistance, and environmental adaptation.
Reference:
- Fux, Alexandra C., et al. "Heterogeneity of lipopolysaccharide as source of variability in bioassays and LPS-binding proteins as remedy." International Journal of Molecular Sciences 24.9 (2023): 8395. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/ijms24098395