TACA-Targeted ADCs, CAR-Ts, and RICs: A New Wave in Cancer Therapy
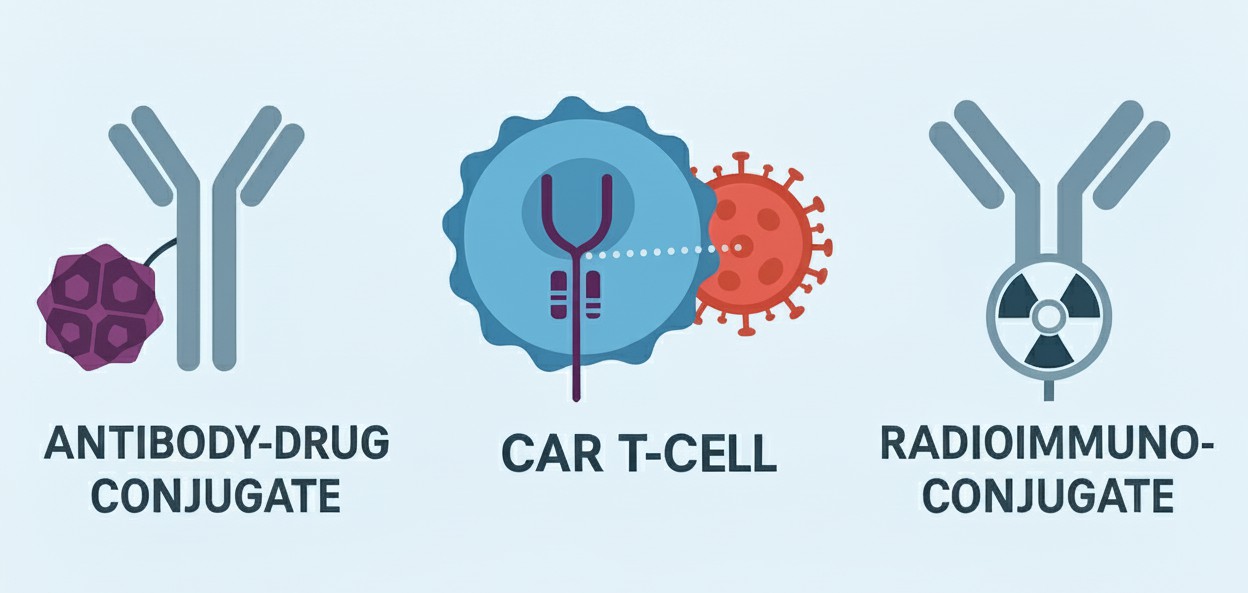
Targeting cancer with monoclonal antibodies (mAbs) is a powerful strategy. However, it faces a significant challenge: many antibody targets are present on both cancer cells and healthy cells. This can cause on-target, off-tumor toxicity. Scientists are now focusing on a more refined class of targets known as Tumor-Associated Carbohydrate Antigens (TACAs). These are abnormal sugar structures, like Tn and sTn, that appear almost exclusively on cancer cells. An even more specific approach is emerging. Instead of targeting just the TACA, new antibodies are designed to bind both the TACA and the protein backbone to which it is attached. This TACA-peptide combination is an excellent, highly specific marker for cancer. This new level of specificity is essential for powerful, next-generation therapies. These therapies include Antibody-Drug Conjugates (ADCs), CAR T-cells, and Radioimmunoconjugates (RICs). Creative Biolabs helps to explore these three exciting therapeutic platforms and how they use TACA-peptide targets to fight cancer. Finding the right antibody for these advanced systems is challenging, but it is crucial to success. Our custom TACA antibody development services are designed to create high-specificity antibodies tailored for these exact applications.
Key Technologies Enabling Glycopeptide-Targeted Therapies
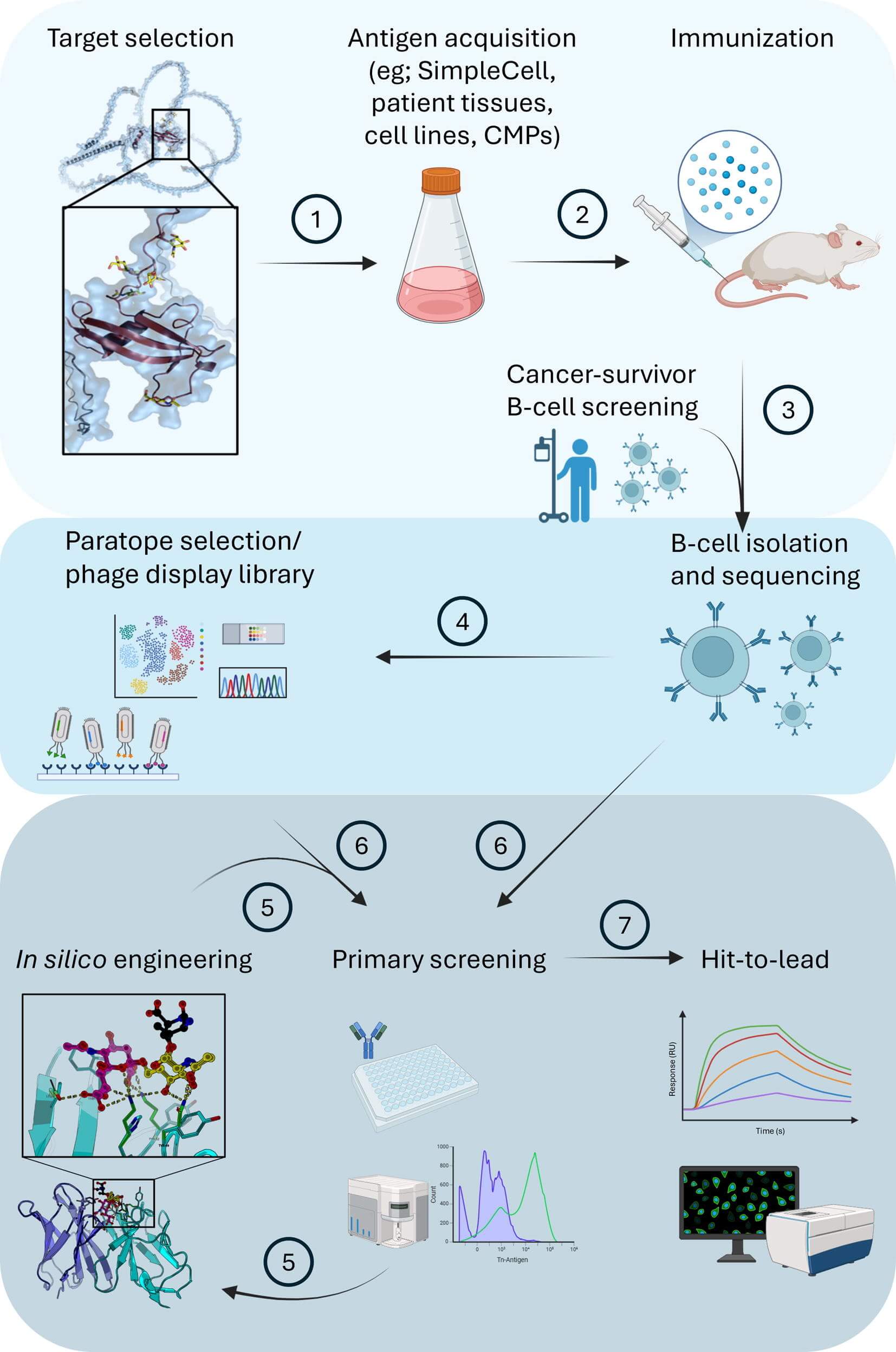 Fig.1 The discovery and optimization workflow
Fig.1 The discovery and optimization workflow
for anti-TACA antibodies.1
The promise of TACA-peptide epitopes is high, but they are tough to target. Success hinges on a few key technologies. First is advanced antigen generation. To find an antibody that recognizes only the cancer form, one must first design the perfect target. Technologies like SimpleCell enable the genetic engineering of cells to produce proteins displaying only the desired truncated glycans, such as Tn or sTn. This allows not just immunization, but also the critical screening step to filter out any antibody that binds to healthy, normally glycosylated proteins. Our custom protein glycosylation platforms are built to solve this exact challenge. With a proper antigen, the next step is discovery. Advanced methods are now preferred over traditional techniques. These include innovative phage display library strategies and screening B-cells directly from cancer survivors, who may have already produced potent anti-TACA antibodies. Finally, structural biology provides the blueprint for success. By studying the crystal structures of antibodies like 5E5 and L2A5, researchers can gain insight into how they bind to the glycopeptide. This information is vital for rationally designing next-generation therapeutics.
Key TACA Targets Summary
To help you navigate this complex and rapidly evolving landscape, the table below summarizes the key TACA targets, highlighting their protein context, examples of therapeutics, and the specific Creative Biolabs services that can accelerate your research for each target.
| TACA Target | Key Protein Context | Example Therapies / Binders | Therapeutic Modality | Relevant Service Solutions |
|---|---|---|---|---|
| Tn (Thomsen-nouveau) |
MUC1 CD44v6 |
5E5 (construct base) CART-TnMUC1 4C8 |
CAR-T | Custom Anti-Tn Antibody Development Service |
| sTn (Sialyl-Tn) |
Ovarian Carcinoma (general) MUC1 |
ST1 ADC L2A5 |
ADC mAb |
Custom Anti-sTn Antibody Development Service |
| TA-MUC1 (Glycopeptide) | MUC1 (Tumor-Associated) |
PankoMab-GEX GT-00A GT-00AXIL15 |
mAb (Clinical) ADC / Immunocytokine |
Custom Anti-TACA Antibody Development Service |
| GD2 (Ganglioside) | Glycosphingolipid |
Naxitamab Dinutuximab GD2 CAR-T |
mAb (Approved) CAR-T (Clinical) |
Custom Anti-Ganglioside Antibody Service |
| Globo H | Glycosphingolipid |
OBI-999 OBI-888 |
ADC (Clinical) mAb (Clinical) |
Custom Anti-Glycolipid Antibody Development |
| Lewis Y (LeY) | Glycan |
SGN-15 Hu3S193 GT-001 |
ADC (Clinical) RIC (Clinical) |
Custom Anti-Lewis Y (LeY) Antibody Development |
| sLeA (CA19-9) | Glycan | HuMab-5B1 (MVT-5873) | mAb (Clinical) | Custom Anti-sLeA (CA19-9) Antibody Development |
| TAG-72 (T/sTn-presenting) | Tumor-Assoc. Glycoprotein |
B72.3 CC49 |
Diagnostic RIC (Preclinical/Clinical) |
Custom Anti-T/sT Antibody Development Service |
| Podocalyxin (Glycopeptide) | Podocalyxin (Tumor-Specific) | PODO447 | mAb / ADC (Preclinical) | Protein Glycosylation Analysis Service |
| CD43 (Glycopeptide) | CD43 (Sialylated Epitope) | KBA1413 | mAb / BiTE (Preclinical) | Custom Anti-TACA Antibody Development Service |
| TF-LYPD3 / TF-CD24 |
LYPD3 CD24 |
GT-002 GT-008 |
mAb / RIC (Preclinical) | Custom Glycosylation of Peptides (for antigen generation) |
Antibody-Drug Conjugates (ADCs)
How TACA-Targeted ADCs Work
Antibody-Drug Conjugates (ADCs) are powerful therapies made of two parts. They link a highly specific antibody to a potent cancer-killing drug (a payload). The antibody's job is to find the cancer cell and bind to it. Once bound, the ADC is taken inside the cell, where the payload is released. This kills the cancer cell directly from the inside. The success of an ADC depends entirely on the specificity of the antibody. The antibody must bind only to cancer cells. If it binds to healthy cells, the toxic payload will harm the patient. TACA-peptide epitopes are excellent targets for ADCs because they are found almost exclusively on tumors, which improves safety and efficacy.
Case Study: ST1 Targets Sialyl-Tn (sTn)
A great example is the ST1 ADC from Siamab Therapeutics, which targets the sTn TACA. ST1 is a humanized antibody linked to a potent drug payload called MMAE. In laboratory studies, this ADC was highly effective in killing sTn-positive tumor cells. In animal models of ovarian cancer, ST1 shrank tumors effectively with no observed toxicity or weight loss. The anti-sTn antibody also appears to reduce a type of immune-suppressing cell (MDSC) in the tumor, which could provide a second way to fight the cancer.
Other TACA-ADCs in Development
Many other TACA-targeted ADCs are also being studied:
- Globo H: OBI-999 is an ADC that targets the glycosphingolipid Globo H. The study revealed that some patients with advanced solid tumors experienced stable disease in a Phase I/II trial.
- Lewis Y (LeY): An ADC named SGN-15 targeted the LeY TACA. In a Phase II trial for lung cancer, it showed a more prolonged median survival for patients, although its development was subsequently paused.
- TA-MUC1: The glycopeptide-specific antibody GT-00A is being developed as an ADC, highlighting its potential for targeted payload delivery.
Chimeric Antigen Receptor (CAR) T-Cell Therapy
Using T-Cells to Target TACAs
Chimeric Antigen Receptor (CAR) T-cell therapy is a powerful treatment. It uses a patient's own immune cells. First, T-cells are collected from the patient. Second, they are genetically engineered in a lab to express a new (chimeric) receptor. This CAR directs the T-cells to find and kill cancer cells. This therapy is highly effective for certain types of blood cancers. However, it has been less successful for solid tumors, primarily because it is challenging to identify targets that are exclusively present on the cancer. TACAs and TACA-peptide epitopes are the ideal solution. They are precise to solid tumors, which can make CAR-T therapy safer and more effective.
Targeting Tn-MUC1
MUC1 is a protein found on many healthy cells, but on cancer cells, its sugar coating is incomplete. This creates a unique TACA-peptide target, designated as Tn-MUC1. Research has shown that CAR-T cells designed to target Tn-MUC1 can effectively control the growth of adenocarcinoma in models. This work has progressed to Phase I clinical trials. This trial utilizes a novel CAR design to prolong the activity of T-cells and mitigate a common issue of exhaustion. Early results are very positive, showing tumor shrinkage and stable disease without severe toxicity.
Targeting Tn-CD44v6
Another new glycopeptide target is Tn-CD44v6, a specific version of the CD44 protein found on cancers. An antibody named 4C8 was developed to bind this Tn-CD44v6 target with high affinity. When 4C8 was used to create a CAR, the resulting CAR-T cells demonstrated highly selective killing of cancer cells, resulting in tumor regression in animal models.
Targeting GD2
TACA-CARs can also target glycolipids, in addition to glycoproteins. A Phase I trial is testing CAR-T cells that target the ganglioside GD2 in patients with a type of brain tumor (glioma). The results have been impressive, with several patients showing neurological improvement and tumor shrinkage.
Radioimmunoconjugates (RICs)
Using TACA Antibodies for Targeted Radiation
Radioimmunoconjugates (RICs) are a third advanced platform. This method uses a monoclonal antibody to deliver a radioactive isotope directly to a tumor. This approach, also known as radioimmunotherapy, concentrates radiation at the cancer site. This maximizes the killing of tumor cells while minimizing radiation exposure to healthy organs. The therapy can use different types of isotopes. These include beta-emitters, such as Lutetium-177 (177Lu), and very powerful alpha-emitters, such as Actinium-225 (225Ac). Just like with ADCs, the safety and success of an RIC depend on the antibody's high specificity for the tumor.
Examples of TACA-Targeted RICs
- TAG-72: The Tumor-Associated Glycoprotein 72 (TAG-72) presents TACA epitopes and is a well-known target. The antibody CC49, which targets TAG-72, has been tested with both 177Lu and 225Ac isotopes.
- TF-CD24: The GT-008 antibody, which recognizes a TACA-peptide epitope (Thomsen-Friedenreich antigen on CD24), is being developed for RIC applications.
- MUC1: Researchers are also actively investigating RICs that use 225Ac to target the abnormal TA-MUC1 epitope.
The field of TACA-targeted cancer therapy is clearly moving beyond simple antibodies. Advanced platforms, such as ADCs, CAR-T cells, and RICs, offer powerful new ways to combat cancer. These therapies can deliver highly potent toxins, engineered immune cells, or radiation directly to the tumor. The success of all these advanced therapies depends on one single factor: specificity. The ability to target unique TACA-peptide epitopes is what finally allows these potent therapies to kill cancer cells while sparing healthy tissues. Creating these highly selective antibodies is a significant challenge. It requires expert antigen design, powerful discovery technologies, and precise analysis of antibody glycosylation. This is precisely where Creative Biolabs can help. We offer a comprehensive range of services tailored to your TACA-targeted projects. We can help you design custom sTn-glycopeptide antigens, discover high-affinity antibodies, and even optimize your antibody's own glycosylation for better function. Are you developing a next-generation TACA-targeted therapeutic? Contact our expert scientific team today to discuss your project and learn how our services can help you reach your goals.
Reference:
- Meier, Edward PW, and Andreas H. Laustsen. "Advances in antibody-based strategies for targeting cancer-associated glycopeptide antigens." Drug Discovery Today (2025): 104507. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1016/j.drudis.2025.104507
Supports
- TACAs Overview
- Guide to Blood Group Antigens
- Comparing sLeA and sLeX Roles in Cancer
- CA19-9 as a Pancreatic Cancer Biomarker
- Lewis Antigen System Overview
- TACA-Targeted ADCs, CAR-Ts, and RICs
