Tumor-Associated Carbohydrate Antigens (TACAs) Overview
One of the most challenging and compelling fields of research is the study of tumor-associated carbohydrate antigens, also known as TACAs. Cancer cells are uniquely adept at evading immune system detection. They achieve this by altering their cell-surface layer—the complex array of molecules that dictates cellular identity. This process, aberrant glycosylation, is a hallmark of cancer. It is caused by the abnormal expression and activity of glycosyltransferases, the enzymes that build glycan chains. This leads to the truncation of O- or N-glycans, the extension of novel chains, or the creation of new glycan structures. The result is a dense layer of tumor-associated carbohydrates. These are not passive structures; they are functional biomolecules that actively contribute to malignancy, promote metastasis, and suppress host immune responses. They are found in a wide variety of cancers, including breast, lung, colorectal, liver, pancreatic, and prostate cancers, as well as melanoma and neuroblastoma. With several decades of collective experience in biotechnology and drug discovery, Creative Biolabs has closely monitored the evolving understanding of cancer biology. We have developed specialized platforms to address this complex field, offering key services such as in-depth glycosylation analysis and robust anti-TACA antibody development. We leverage this deep expertise to define the fundamental concepts, explore the structure of TACAs, their functional roles, and their use in diagnostics, as well as the advanced strategies being developed to target them for research and therapeutic development.
The Core Concept: Tumor-Associated Carbohydrate Antigens (TACAs)
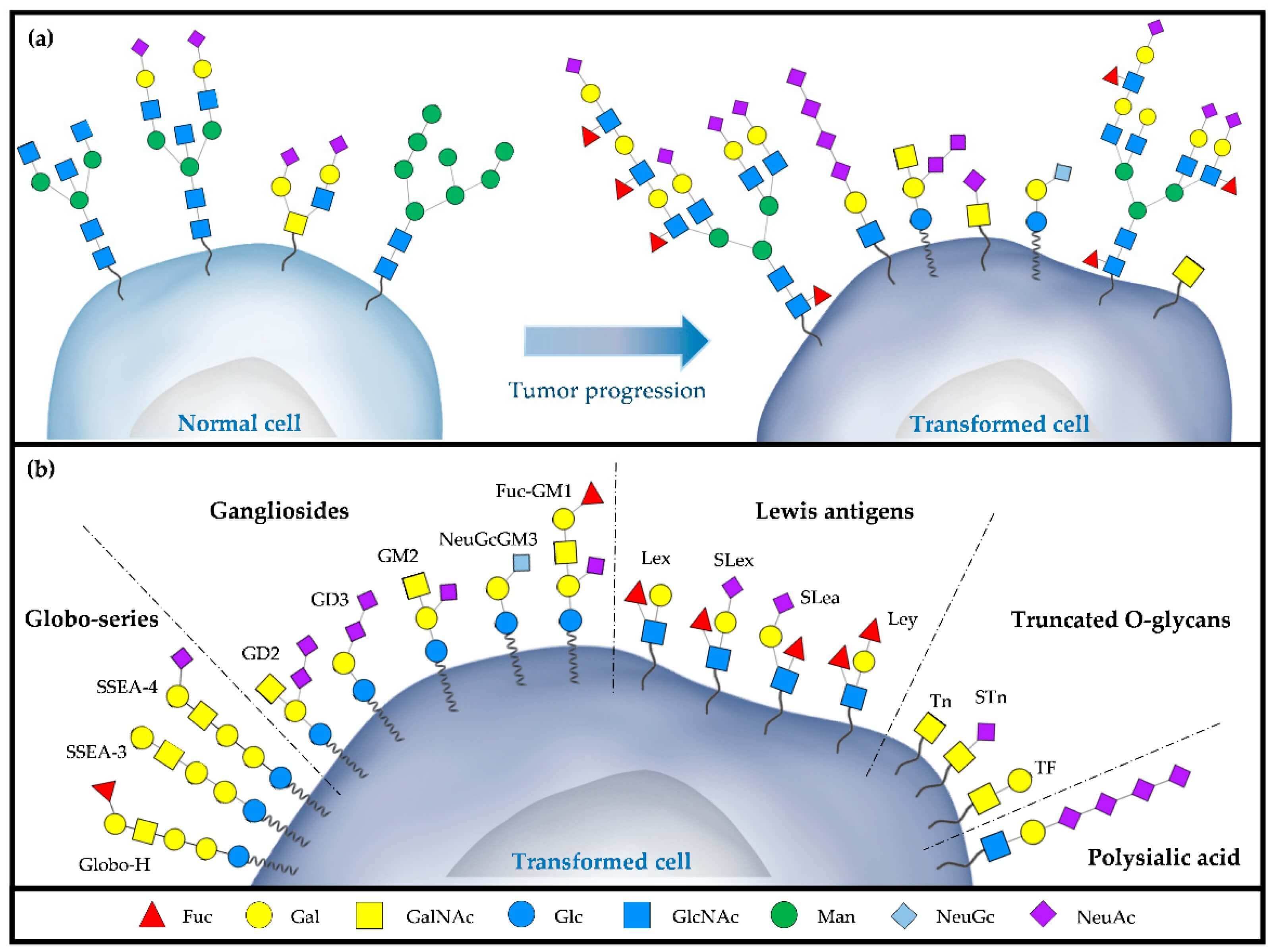 Fig.1 Schematic of (a) aberrant glycosylation during tumor progression
Fig.1 Schematic of (a) aberrant glycosylation during tumor progression
and (b) major TACAs expressed on a tumor cell.
To understand this specific class of Tumor-Associated Antigen (TAA), we must first answer: what is a carbohydrate antigen? Simply put, it is an antigen where the immune-recognized epitope is a carbohydrate structure (a glycan chain). The most famous example is the ABO blood group system; the A, B, and O antigens are carbohydrate antigens on the surface of your red blood cells. With that established, we can define Tumor-Associated Carbohydrate Antigens (TACAs). These are specific carbohydrate structures that are preferentially expressed by cancer cells due to aberrant glycosylation. They are a distinct and highly important subclass of TAAs. TACAs do not exist in isolation. They are covalently attached to "scaffold" molecules on the cell membrane, forming glycoconjugates.
- Glycoproteins: The TACA is attached to a protein. The most extensively studied example is Mucin-1 (MUC1), a large glycoprotein that is overexpressed and aberrantly glycosylated in many epithelial cancers. In cancer, its O-glycan chains are severely truncated, exposing dense clusters of TACAs.
- Glycolipids: The TACA is attached to a lipid (ceramide) anchored in the membrane. These are known as gangliosides or glycosphingolipids.
A Table of Major TACAs in Research
TACA expression is highly heterogeneous, varying between cancer types and even between patients with the same cancer. However, certain TACAs are strongly associated with specific malignancies.
| TACA Name | Classification | Core Structure | Associated Cancers (Research) |
|---|---|---|---|
| Tn Antigen | O-glycan | Truncated | Breast, Prostate, Stomach, Colorectal |
| Sialyl-Tn (sTn) | O-glycan | Truncated | Breast, Ovarian, Pancreatic, Colorectal |
| T Antigen | O-glycan | Truncated | Breast, Colon, Bladder |
| Sialyl-Lewis A (sLeA) | Lewis-type | Glycolipid/Protein | Pancreatic, Colorectal, Gastric, Breast |
| Sialyl-Lewis X (sLeX) | Lewis-type | Glycolipid/Protein | Pancreatic, Lung, Breast, Colon |
| GD2 | Ganglioside | Glycolipid | Neuroblastoma, Melanoma, Small-cell Lung Cancer |
| GD3 | Ganglioside | Glycolipid | Melanoma, Glioblastoma |
| GM2 | Ganglioside | Glycolipid | Melanoma, Sarcoma, Brain Tumors |
| Lewis Y (LeY) | Lewis-type | Glycolipid/Protein | Breast, Prostate, Ovary, Lung |
| Globo H | Glycosphingolipid | Glycolipid | Breast, Prostate, Ovary |
| MUC1 (glycoform) | Mucin | Glycoprotein | Breast, Pancreatic, Ovarian, Lung |
TACAs in Cancer Diagnosis and Prognosis
Beyond their biological function, TACA expression has significant clinical implications.
Role in Diagnostics
For decades, the detection of shed TACAs or TACA-carrying proteins in patient serum has been a cornerstone of cancer diagnostics and monitoring. Several key biomarkers, such as CA 19-9 (a structure related to sLeA, used for digestive tract/pancreatic cancer), CA 15-3 (related to MUC1, for breast cancer), and CA 125 (for ovarian cancer), are all based on detecting TACA-associated glycoproteins. TACA expression can correlate with disease stage. For example, sLeA expression is used in research to help stage pancreatic cancer.
Value as Prognostic Markers
TACA expression on a tumor biopsy often provides powerful prognostic information. The presence of specific TACAs is usually associated with a more aggressive disease phenotype. For example, the expression of sLeA, sTn, or sLeX in breast and other cancers is often correlated with increased lymph node metastasis and reduced overall survival.
The Functional Roles of TACAs in Cancer Progression
Aberrant glycosylation is actively selected for because it provides distinct survival advantages. TACAs are functional effectors that directly promote the hallmarks of cancer.
Role 1: Immune Evasion (Glyco-Immune Checkpoints)
This is a primary function. TACAs are highly effective at suppressing immune cell activity. They function as ligands for inhibitory receptors on immune cells, particularly the Siglec (Sialic acid-binding immunoglobulin-like lectin) family. Many TACAs are heavily sialylated. Infiltrating immune cells (T-cells, NK cells, macrophages) express inhibitory Siglec receptors. The binding of sialylated TACAs to these Siglec receptors transmits a powerful inhibitory signal directly into the immune cell, paralyzing its cytotoxic function. This allows the TACA-expressing tumor cell to create its own "glyco-immune checkpoint," shielding it from immune system attacks.
Role 2: Metastasis (Adhesion and Extravasation)
Metastasis is the primary cause of cancer-related mortality. TACAs are essential mechanical components of this process. For a circulating tumor cell to metastasize, it must adhere to the vascular endothelium (the blood vessel wall) of a new organ. Endothelial cells express adhesion molecules called selectins. Cancer cells upregulate TACA ligands for these selectins, most notably Sialyl Lewis X (sLeX) and Sialyl Lewis A (sLeA). This TACA-selectin binding mediates the initial "tethering" and "rolling" of the CTC, allowing it to adhere, exit the bloodstream (extravasate), and initiate a new metastatic colony.
Role 3: Proliferation and Signaling
TACAs are physically attached to the very receptors that control cell growth, such as receptor tyrosine kinases (RTKs) like EGFR or HER2. Aberrant glycosylation of these receptors can fundamentally alter their function, promoting ligand-independent dimerization and activation. This results in a constant, oncogenic "ON" signal driving uncontrolled proliferation.
Role 4: Association with Cancer Stem Cells (CSCs)
Cancer stem cells are the subpopulation of cells responsible for tumor initiation, recurrence, and resistance. The link between TACAs and CSCs is an area of intense research. Some TACAs, particularly Tn and sTn, are enriched in CSC populations in epithelial cancers. The direct functional interaction is not yet fully elucidated. However, the mechanism is likely indirect. We know that TACAs are critical for shaping the tumor microenvironment (TME). It is hypothesized that TACAs modulate TME signaling pathways that are, in turn, essential for maintaining the "stemness" and survival of CSCs.
Current Strategies for Targeting TACAs
Overcoming these complex challenges is a primary goal of modern glycobiology. At Creative Biolabs, we support our clients as they deploy sophisticated strategies to target TACAs.
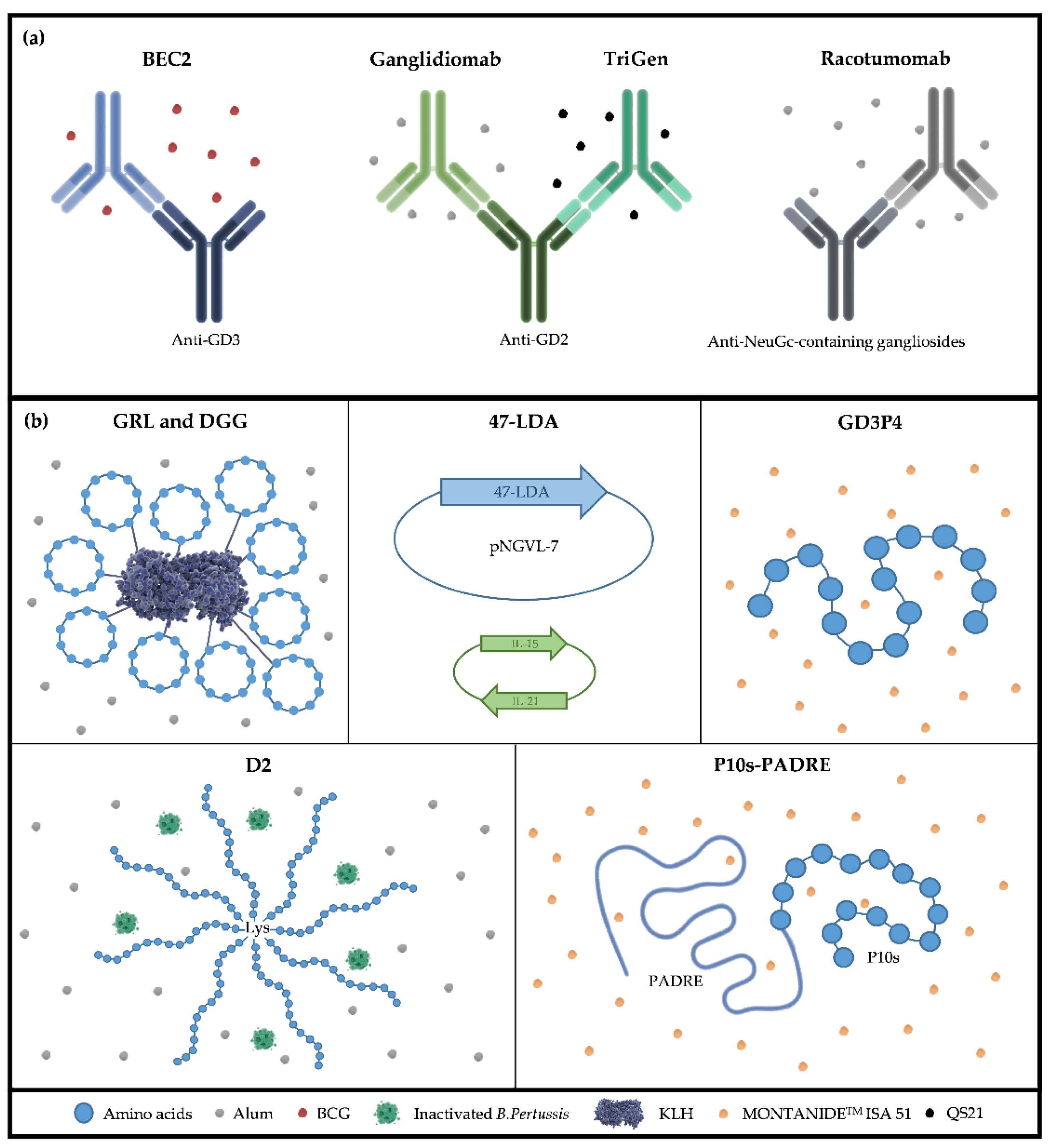 Fig.2 Active immunotherapy strategies targeting TACAs, including (a) anti-idiotype antibodies and (b) peptide mimetics.
Fig.2 Active immunotherapy strategies targeting TACAs, including (a) anti-idiotype antibodies and (b) peptide mimetics.
Strategy 1: TACA-Based Vaccines
The objective is to educate the patient's own immune system to attack TACA-expressing cells. To overcome poor immunogenicity, the TACA (as a hapten) is chemically linked to a large, immunogenic carrier protein. This conjugate vaccine is delivered with a powerful adjuvant. This formulation utilizes the carrier protein to facilitate T-cell help, thereby driving a T-cell-dependent B-cell response and resulting in the production of high-affinity anti-TACA IgG antibodies. Research is also focused on "non-self" mimics of TACAs and polyvalent vaccines that target multiple TACAs simultaneously. Despite decades of research, no TACA-based vaccine has yet received FDA approval, highlighting the challenges associated with this approach.
Strategy 2: Monoclonal Antibodies (mAbs)
This strategy administers pre-made, high-potency anti-TACA antibodies. This approach has yielded a major clinical victory. An anti-GD2 monoclonal antibody is FDA-approved and a standard of care for treating high-risk neuroblastoma. This success proves that TACAs are viable and druggable targets.
Strategy 3: Advanced Therapeutic Formats
Building on the success of monoclonal antibodies (mAbs), researchers are engineering more complex and potent biotherapeutics.
- CAR-T Cell Therapy: T-cells are engineered with a Chimeric Antigen Receptor (CAR), often built from an antibody, that allows the T-cell to "see" and "bind" a TACA directly. Anti-GD2 and anti-MUC1 CAR-T cells are in active clinical and preclinical development.
- Antibody-Drug Conjugates (ADCs): An anti-TACA mAb is used as a targeting vehicle to deliver a highly potent cytotoxic payload directly to the tumor cell.
- Bispecific Antibodies (BsAbs): These antibodies have two binding domains: one arm binds the TACA on the tumor cell, and the other binds an activating receptor on a T-cell, physically forcing the immune cell to engage and kill the tumor.
Strategy 4: Combination and Multi-Targeted Therapies
The future of TACA-based treatment likely lies in rational combinations. The complexity of cancer biology requires a multi-pronged attack. Targeting a single pathway is often insufficient. Synergistic effects can be achieved by combining TACA-targeted therapies with other treatments. Here are some examples:
- TACA-Therapy + Checkpoint Inhibition: A TACA-targeted therapy could kill tumor cells, while an immune checkpoint inhibitor (e.g., anti-PD-1) releases the "brakes" on the T-cells, enabling a more robust and widespread immune attack.
- TACA-Therapy + Small Molecule Inhibitors: Combining a TACA-targeted agent with an inhibitor of a key oncogenic pathway (like the PI3K/Akt or Raf/MEK/ERK pathways) can simultaneously attack the tumor from the outside (immunologically) and the inside (signal transduction).
- TACA Biosynthesis Inhibitors: Another approach is to block the glycosyltransferases that create the TACAs, thereby altering the cell's phenotype and potentially reducing its malignancy.
How Creative Biolabs Advances TACA Research
At Creative Biolabs, we are deeply committed to advancing this field. We provide the specialized tools and services that researchers require to overcome the distinct challenges of TACA-based R&D.
Specialized Anti-TACA Antibody Development
Generating high-affinity, high-specificity antibodies against TACAs is a well-known technical hurdle. We have developed specialized platforms to address the most critical TACA targets.
- Anti-Glycolipid Antibody Development: We leverage unique strategies to develop antibodies against complex glycolipid targets like GD2, GD3, and GM2, which are often poorly immunogenic.
- Anti-MUC1 Glycan Antibody Development: Targeting the aberrant glycoforms of MUC1 (such as Tn and sTn) requires precise targeting. Our platforms are designed to generate antibodies specific to these glycopeptide epitopes.
Glycosylation Analysis and Engineering
We provide services to help build and analyze next-generation therapeutics.
- Glycosylation Analysis Service: We offer comprehensive services to precisely characterize the complex glycan structures on your biomolecules, which is essential for understanding TACA expression or optimizing a therapeutic.
- Custom Glycosylation Services: Our team can custom-engineer specific glycoforms on various biomolecules, enabling the creation of specific TACA-based screening tools, vaccines, and ADCs.
Glycoarray Platform
Verifying the specificity of a new therapeutic is crucial to avoid the safety risks associated with off-target binding. We have developed a comprehensive suite of glycoarray services. This platform enables our clients to precisely characterize the binding profile of their antibodies against an extensive panel of tumor-associated carbohydrates.
TACAs represent one of the most complex and enduring challenges in cancer biology. For many years, they were considered intractable targets due to immunological tolerance and poor immunogenicity. This perspective is now changing. We now have a clear understanding that TACAs are not merely markers, but active and critical mediators of tumor progression, immune evasion, and metastasis. While the road to a successful TACA-based vaccine remains long, the landmark FDA approval of an anti-GD2 monoclonal antibody has provided definitive proof of principle: TACAs are druggable targets. The future lies in combining our advanced discovery platforms with a deeper understanding of tumor heterogeneity to create personalized and synergistic combination therapies. We at Creative Biolabs are proud to partner with researchers worldwide to help turn this carbohydrate shield, which cancer uses as its most excellent defense, into its greatest vulnerability. Please feel free to contact us for more information about our services.
Reference:
- Segatori, Valeria Inés, et al. "Mimicry of Tumour-Associated Carbohydrates: Is It a Promising Option for Cancer Treatment?" Immuno 3.2 (2023): 122-147. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/immuno3020009
Supports
- Understanding Glycosylation
- Glycosylation Influences Blood Type
- Anti-Glycolipid Antibody Overview
- GM3 Ganglioside Overview
- Sulfatide and Anti-Sulfatide Antibodies Overview
- TACAs Overview
- Guide to Blood Group Antigens
- Comparing sLeA and sLeX Roles in Cancer
- CA19-9 as a Pancreatic Cancer Biomarker
- Lewis Antigen System Overview
- TACA-Targeted ADCs, CAR-Ts, and RICs
