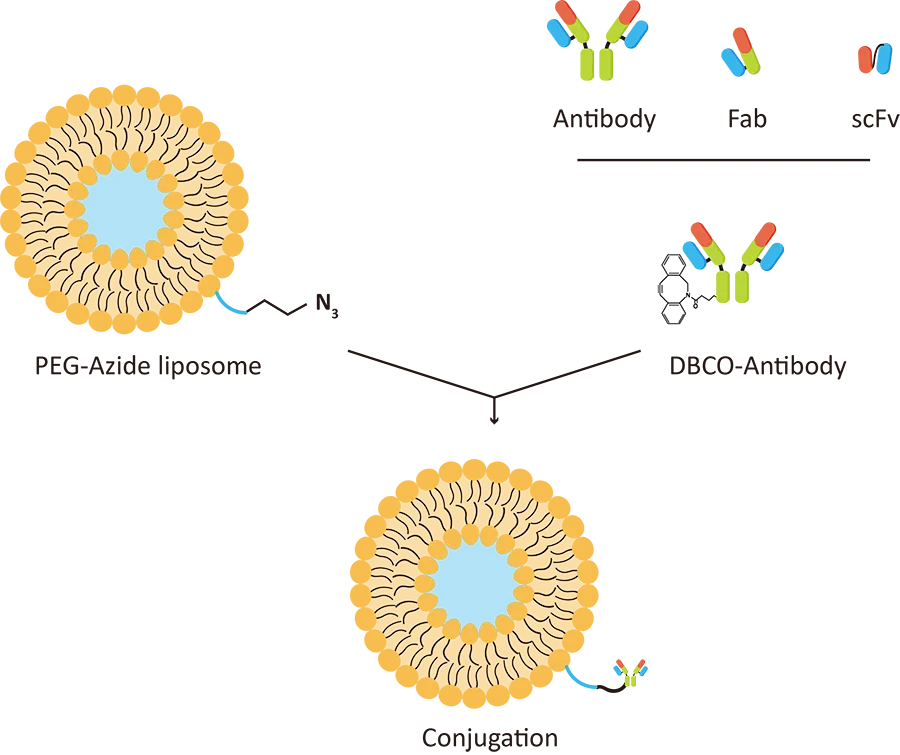
Lipid Nanoparticle Synthesis Service
Build reproducible, high-performance lipid nanoparticles (LNPs) tailored to your RNA, DNA, or protein payload—designed, synthesized, and analytically qualified end-to-end by Creative Biolabs.
Trusted by Industry Leaders
Creative Biolabs supports biopharma and advanced academic labs with formulation design, microfluidic manufacturing, and deep characterization of state-of-the-art LNP systems.





Why High-Fidelity LNP Synthesis Matters
Lipid nanoparticles have become the leading non-viral vector for nucleic acids because they enable efficient encapsulation, protect labile payloads, and promote intracellular delivery. Standard four-component LNPs—an ionizable lipid, a helper phospholipid, cholesterol, and a PEG-lipid—consistently achieve high encapsulation and tunable biodistribution when their composition and process parameters are optimized together.
Yet, small changes—ionizable lipid pKa, PEG-lipid anchor length, or the microfluidic mixing regime—can shift potency, stability, and size distribution. Getting those dialed in requires integrated formulation science, precise synthesis, and rigorous analytics. Creative Biolabs provides this full stack so your LNPs perform as designed, batch after batch.
What We Do: LNP Formulation, Synthesis, and Analytics
Formulation Design & Lipid Selection
- Ionizable Lipid Strategy: We select or screen ionizable lipids with apparent pKa in the optimal window for endosomal escape and low systemic charge at neutral pH.
- Helper Lipid & Cholesterol Engineering: DSPC/DOPE and cholesterol content modulate membrane packing, fusogenicity, and structural order.
- PEG-Lipid Architecture: We vary PEG MW and anchor length, plus cleavable/ss-linker options, to control colloidal stability and protein corona formation.
- Charge Ratio (N/P) And Composition Maps: We design DoE spaces to co-optimize encapsulation efficiency, size, and potency for your specific payload.
Microfluidic Synthesis & Process Development
- Platform Mixers: From staggered herringbone micromixers (SHM) to impinging/coaxial jet devices, we select the optimal mixing geometry.
- Process Knobs That Actually Matter: We explicitly control flow-rate ratio (FRR), total flow rate (TFR), lipid concentration, and temperature to shape nucleation and growth.
- Scale-Up With Quality By Design: We translate lab conditions to larger mixers while maintaining critical quality attributes (CQAs) using DoE, PAT, and ICH-aligned control strategies.
Deep Characterization & Release Testing
- Physical Attributes: DLS/NTA for size/PDI; cryo-TEM for morphology; zeta potential; and osmolality for processing readiness.
- Chemical & Compositional Analytics: Encapsulation efficiency, residual solvent by GC, lipid composition by UHPLC-MS, and nucleic acid integrity.
- Functional Readouts (Research-Only): In-vitro transfection or silencing panels and serum stability profiling.
- Stability Studies: Accelerated/real-time, freeze–thaw robustness, and stress testing.
Why Choose Creative Biolabs for LNP Synthesis
Our platform integrates advanced formulation science with precise microfluidic synthesis to provide you with superior, efficient, and reliable LNP production services.
Evidence-Based Formulation Design
Leverages the latest research on ionizable lipid pKa, helper lipids, cholesterol, and PEG-lipid dynamics.
Precise Microfluidic Control
Tunes FRR/TFR and other process parameters for exact size and PDI targets.
Seamless Scale-Up
Maintains critical quality attributes from lab scale to larger or continuous manufacturing.
Comprehensive Analytics
Provides complete characterization and defensible data packages with every batch.
Flexible Payload Capability
Supports mRNA, saRNA, gRNA/SpCas9 mRNA, ASOs, and plasmids.
Dedicated Scientific Support
Offers project-specific guidance from initial design to final deliverables.
Applications of LNP Synthesis
mRNA Expression Studies
Build LNPs for reporter or protein-of-interest expression in cell lines or primary cells, optimizing for uptake and endosomal escape.
Gene Editing Co-Delivery
Configure LNPs for SpCas9 mRNA + sgRNA or RNPs, using FRR/TFR to hit size windows that favor cellular uptake.
Biodistribution Method Development
Explore how PEG chain length/anchor and cholesterol content influence circulation and organ partitioning.
Process Development Models
Prototype continuous or intensified workflows under ICH Q13 concepts and define PAT checkpoints for future tech transfer.
How We Build Your LNPs: A Clear Workflow
Project Intake & Target Definition
Define payload (mRNA/saRNA/gRNA/ASO/protein), intended route and matrix, particle size/PDI targets, and assay plan.
Formulation Design & DoE Mapping
Develop a formulation hypothesis and map ionizable lipid, helper lipid, cholesterol, PEG-lipid, and N/P design space.
Microfluidic Method Selection & Setup
Choose SHM, impinging jet, or coaxial jet mixer based on scale and CQAs; lock FRR/TFR and key process parameters.
Rapid Screening & Optimization
Conduct mini-batch iterations to converge on size, PDI, EE%, and preliminary potency; refine variables for optimal performance.
Scale Translation & Stability Verification
Confirm reproducibility at higher TFRs; verify robustness through in-process controls; complete stability testing and analysis.
Reporting, Tech Transfer & Final Deliverables
Deliver a comprehensive data set, SOPs, and recommended control strategy, enabling seamless transition to in-house or CDMO manufacturing.
What You Receive: Concrete Deliverables
- ✓Formulation set: 2–5 optimized LNP formulations with defined lipid ratios, N/P values, and target size/PDI.
- ✓Pilot batches: Multi-hundred-milligram (or project-specific) lots with retains.
- ✓Analytics dossier: DLS/NTA, zeta potential, EE%, solvent residues, lipid profile, nucleic acid integrity, cryo-TEM (if scoped), and stability data.
- ✓Process packet: Mixer setup, FRR/TFR ranges, CPP/CQA definitions, in-process checks, and PAT endpoints for continuous manufacturing.
- ✓Assay summary: In-vitro expression/silencing tests and serum interaction notes.
All services are strictly For Research Use Only. Not For Clinical Use.
Related LNP And Lipid Services From Creative Biolabs
To further support your lipid-based delivery research, Creative Biolabs offers a full spectrum of complementary services that seamlessly integrate with our lipid nanoparticle synthesis expertise. These solutions help you explore new formulations, expand delivery strategies, and accelerate your development pipeline with the same scientific rigor and quality standards.
Frequently Asked Questions
LNP synthesis is the creation of nanoscale lipid spheres that encapsulate molecules like mRNA or DNA. Methods include thin-film hydration, high-energy homogenization, and advanced microfluidics, offering precise control, scalability, and high encapsulation efficiency for sensitive payloads.
Other Resources
References
- Hou, Xucheng, et al. "Lipid nanoparticles for mRNA delivery." Nature Reviews Materials 6.12 (2021): 1078-1094. https://doi.org/10.1038/s41578-021-00400-1
- Hamilton, Nicholas B., et al. "Calculating Apparent p K a Values of Ionizable Lipids in Lipid Nanoparticles." Molecular pharmaceutics 22.1 (2024): 588-593. https://doi.org/10.1021/acs.molpharmaceut.4c00426
- O'Brien Laramy, Matthew N., et al. "Process robustness in lipid nanoparticle production: a comparison of microfluidic and turbulent jet mixing." Molecular pharmaceutics 20.8 (2023): 4285-4296. https://pubs.acs.org/doi/10.1021/acs.molpharmaceut.3c00390
Online Inquiry
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.