Creative Biolabs has been dedicated to liposome development for many years. Our liposomal transfection reagent is renowned for its outstanding transfection efficiency, ensuring optimal results for your experiments.
Cationic liposome-mediated transfection is one of the most common methods for introducing exogenous genetic material into cells. Through electrostatic interactions, positively charged cationic liposomes can form complexes with negatively charged nucleic acids. These complexes can be adsorbed by the negatively charged cell membrane and internalized into the cell through endocytosis. Liposome-based transfection offers advantages such as low immunogenicity, high reproducibility, simple operation, and no need for special equipment.
Creative Biolabs provides a range of efficient transfection reagents based on cationic liposomes and LNPs, and our stringent quality control ensures the stability and reliability of our products. You can select the appropriate product based on "Cell Type" and "Sample Type," and feel free to contact us for more detailed information.
Efficient mRNA Delivery with mRNA Lipoplexes Prepared Using a Modified Ethanol Injection Method
Author: Tang, Min, et al.
This project explores the impact of lipid composition, mRNA concentration, and transfection time on transfection efficiency through luciferase expression in hela cells. Researchers prepared 18 lipid mixtures comprising 8 cationic lipids and 3 helper lipids. Subsequently, they prepared cationic liposome/mRNA complexes (mRNA lipoplexes) by combining the lipid mixtures with mRNA. Figure A demonstrates that luciferase expression is significantly higher in lipoplexes with lipid compositions DC-1-14/DOPE, DC-1-16/DOPE, and TC-1-12/DOPE compared to other lipoplex groups. Luciferase activity is optimal when mRNA concentration ranges from 0.5 to 1.0 μg/mL (Figure B) and transfection time is 24 hours (Figure C). This project indicates that transfection reagents based on cationic liposomes exhibit high transfection efficiency, with lipid composition, mRNA concentration, and transfection time significantly influencing transfection efficiency.
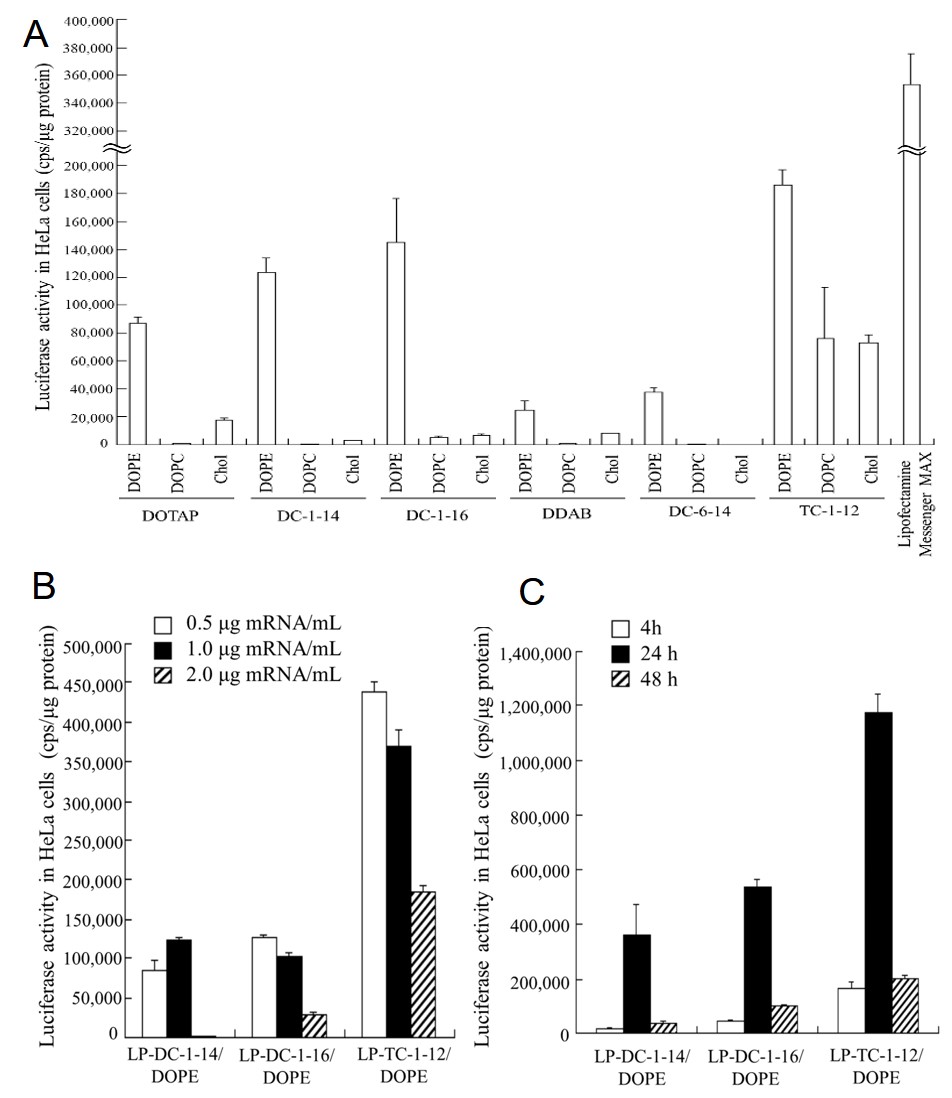 Fig.1 The impact of lipid composition, mRNA concentration, and transfection time on luciferase expression in hela cells.1,2
Fig.1 The impact of lipid composition, mRNA concentration, and transfection time on luciferase expression in hela cells.1,2
References
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical Use| Cat | Product name | Cell Type | Sample Type | Data sheet | MSDS | Inquiry |
|---|---|---|---|---|---|---|
| LDLY-0724-LD127 | Lipofection Reagent-Universal (Liposome) | Eukaryotic cells such as HEK293T, Hela, NIH3T3, HEK293FT, CHO cells | Plasmids, siRNAs, or other forms of nucleic acids. |
|
|
Inquiry |
| LDLY-0724-LD128 | Lipofection 293-Susp (Liposome) | Suspension-cultured HEK293 cells | Plasmid |
|
|
Inquiry |
| LDLY-0724-LD129 | Lipofection 293-Susp Pro (Liposome) | Suspension-cultured HEK293 cells | Plasmid |
|
|
Inquiry |
| LDLY-0724-LD130 | Lipofection 293-Adh (Liposome) | Adherent-growing HEK293, HEK293T, HEK293A, 293FT, etc., in the 293 cell series | Plasmid |
|
|
Inquiry |
| LDLY-0724-LD131 | Lipofection 293-Adh Pro (Liposome) | Adherent-growing HEK293, HEK293T, HEK293A, 293FT, etc., in the 293 cell series | Plasmid |
|
|
Inquiry |
| LDLY-0724-LD132 | Insect Lipofection Reagent (Liposome) | Insect cells such as Sf9 and Sf21 | Baculovirus plasmid (bacmid) DNA |
|
|
Inquiry |
| LDLY-0724-LD133 | Universal mRNA Lipofection Reagent (LNP) | Eukaryotic cells such as HEK293T, WRL-68, HFL-1, A549, MCF7, Capan-2 cells | mRNA |
|
|
Inquiry |
| LDLY-0724-LD134 | mRNA Lipofection Reagent (LNP) | T cells | mRNA |
|
|
Inquiry |
| LDLY-0724-LD135 | Universal siRNA Lipofection Reagent (LNP) | Eukaryotic cells such as HEK293T, WRL-68, HFL-1, A549, MCF7, Capan-2 cells | siRNA |
|
|
Inquiry |
| LDLY-0325-LD493 | Lipofection Reagent Mouse Immune Cell (LNP) | Mouse immune cells | mRNA, siRNA, sgRNA |
|
|
Inquiry |
| LDLY-0325-LD494 | Lipofection Reagent Human Immune Cell (LNP) | Human immune cells | mRNA, siRNA, gRNA, saRNA, circRNA |
|
|
Inquiry |
| LDLY-0325-LD495 | Liver-Targeted Lipofection Reagent (LNP) | mRNA, siRNA, DNA plasmid and other nucleic acid molecules |
|
|
Inquiry | |
| LDLY-0325-LD496 | Spleen-Targeted Lipofection Reagent (LNP) | mRNA, siRNA, DNA plasmid and other nucleic acid molecules |
|
|
Inquiry |
Online Inquiry