ABCC9 forms KATP channels in vascular smooth muscle, coupling ATP/ADP ratios to tone. Hypoxia/ischemia triggers ADP-induced channel opening, causing hyperpolarization and vasodilation.
Introduction to ABCC9
ABCC9 (ATP-Binding Cassette Subfamily C Member 9), also known as SUR2 (Sulfonylurea Receptor 2), is a critical component of the ATP-sensitive potassium (KATP) channel complex. This transmembrane protein regulates ion channel activity by sensing intracellular ATP/ADP ratios, linking cellular metabolism to electrical excitability. Mutations in ABCC9 are associated with Cantu syndrome (hypertrichosis, cardiac abnormalities) and dilated cardiomyopathy, while its dysregulation contributes to metabolic disorders, including diabetes and obesity. ABCC9's role in vascular smooth muscle and pancreatic β-cells further positions it as a therapeutic target for cardiovascular and metabolic diseases.
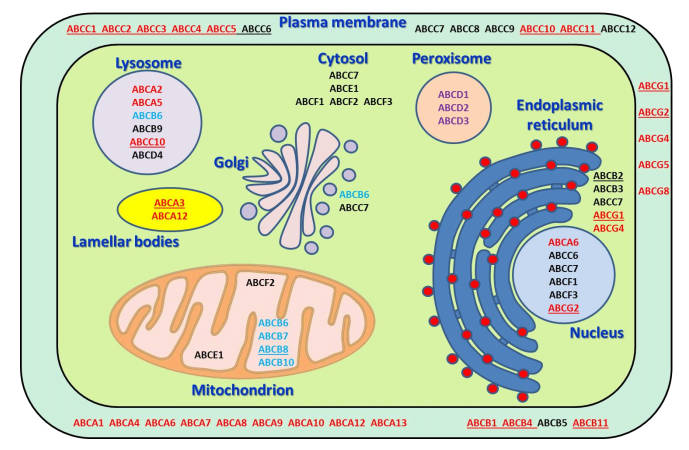 Fig.1 Schematic depiction of localization of ABC transporters within the cell and their major functions.1
Fig.1 Schematic depiction of localization of ABC transporters within the cell and their major functions.1
Key Biological Functions
Cardiovascular: Metabolic Sensing & Vasodilation
Neuronal: Synaptic Modulation & Neuroprotection
ABCC9/Kir6.2 channels in neurons integrate metabolism with excitability. PKC phosphorylation at S104 inhibits opening, enhancing transmission; dysfunction links to epilepsy. Ischemia activates channels, limiting mitochondrial Ca2+ overload and reducing infarct size. ABCC9 decline in the hippocampus correlates with Alzheimer's tau pathology.
Glucose: β-Cell Function & Diabetes
ABCC9 regulates GSIS in β-cells: ATP closes channels, depolarizing membranes to trigger insulin release. Sulfonylureas bind NBD2 to force closure, treating type 2 diabetes.
Cancer: Warburg Effect & Chemoresistance
Tumors exploit ABCC9 to sustain KATP channels, promoting glycolysis and acidifying the microenvironment. Hyperpolarization activates mTORC1, supporting growth. In ovarian cancer, ABCC9 overexpression inhibits paclitaxel-induced apoptosis; inhibitors restore sensitivity.
Featured Technologies for ABCC9 Analysis
RNA Sequencing
Our advanced technology meticulously measures ABCC9 gene expression, shedding light on vital biological pathways. With an experienced team at the helm, expect unparalleled accuracy and reliable data to fuel your research triumphs.
Cryo-Electron Microscopy (Cryo-EM)
Cryo-EM resolves the high-resolution structure of ABCC9 in complex with Kir6.x, uncovering ligand-binding sites and allosteric modulation mechanisms. Structural insights guide rational drug design.
Co-Immunoprecipitation (Co-IP) & Mass Spectrometry
Co-IP coupled with mass spectrometry maps ABCC9's protein interactome, identifying partners like PKC, mTORC1 components, and mitochondrial regulators. These interactions reveal ABCC9's role in cross-talk between ion channel activity and metabolic signaling.
Applications
Cardiovascular Pathophysiology & Therapeutic Targets
ABCC9's role in cardiac KATP channels extends beyond hypertrophy to ischemic preconditioning. Studies show ABCC9 activation during myocardial infarction reduces infarct size by 40% in pigs, while its inhibition exacerbates heart failure in pressure-overload models.
Neurodevelopmental & Psychiatric Disorders
ABCC9 modulates synaptic plasticity in the prefrontal cortex; its dysregulation is implicated in autism spectrum disorder (ASD) and schizophrenia. ABCC9-knockout mice exhibit impaired social recognition and repetitive behaviors, mirroring core ASD symptoms. Human brain organoid studies reveal ABCC9 variants disrupt GABAergic interneuron migration, suggesting channel modulators as potential treatments for neuropsychiatric conditions.
Metabolic Syndrome & Obesity Interventions
ABCC9 in adipose tissue regulates lipolysis and thermogenesis. Obese patients with ABCC9 loss-of-function variants show increased visceral fat accumulation and insulin resistance.
Renal Disease Progression Biomarkers
ABCC9 expression in podocytes correlates with diabetic nephropathy severity. Urinary exosomal ABCC9 mRNA levels predict renal function decline in type 1 diabetes patients, outperforming traditional markers like albuminuria.
FAQs
-
What is ABCC9, and what is its biological function?
ABCC9 encodes the SUR2 subunit of ATP-sensitive potassium (KATP) channels. These channels regulate cellular excitability by coupling metabolic status to membrane potential, playing critical roles in cardiac muscle, pancreatic β-cells, neurons, and smooth muscle. Dysfunction in ABCC9 is linked to cardiovascular diseases, neurological disorders, and metabolic syndromes.
-
How are ABCC9 mutations associated with human diseases?
ABCC9 mutations cause a rare disorder characterized by hypertrichosis, cardiac defects, and facial dysmorphism. Additionally, variants in ABCC9 are associated with arrhythmogenic cardiomyopathy, type 2 diabetes, and epilepsy.
-
How does ABCC9 relate to aging and longevity?
ABCC9 expression declines with age, correlating with mitochondrial dysfunction and vascular stiffness. Findings suggest ABCC9 modulators could delay age-related diseases, such as heart failure and atherosclerosis.
-
What are the future directions in ABCC9 research?
Key areas include:
- Developing small-molecule modulators (agonists/antagonists) for clinical use.
- Elucidating ABCC9's role in immune-metabolic crosstalk (e.g., during infections).
- Using organoids and 3D bioprinting to model ABCC9-related diseases.
- Integrating multi-omics data (genomics, proteomics, metabolomics) to uncover novel pathways.
ABCC9 analysis service empowers researchers to decode the role of KATP channels in health and disease. By integrating genetic, functional, and computational tools, we accelerate discoveries in cardiovascular biology, metabolism, and therapeutics. Contact us today to design a customized ABCC9 study or request a free consultation!
Reference
- Vrana, David, et al. "ABC transporters and their role in the neoadjuvant treatment of esophageal cancer." International journal of molecular sciences 19.3 (2018): 868. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/ijms19030868
For Research Use Only.