B2M serves as an exceptionally sensitive indicator of renal tubular dysfunction, enabling early detection of kidney injury. It is invaluable for monitoring drug-induced nephrotoxicity, assessing the health of kidney transplants, and identifying early tubular damage in conditions like diabetic nephropathy.
At Creative Biolabs, we specialize in beta-2 microglobulin (B2M) analysis, a critical area gaining interest across haematologic malignancies, renal diseases, and chronic inflammatory conditions. Leveraging extensive expertise in biomarker analysis, we offer diverse, advanced assays and platforms, from highly customized solutions to off-the-shelf options, to propel your research and drug development programs forward.
Introduction of B2M
B2M is a low-molecular-weight protein (11.8kDa) that serves as a subunit of the major histocompatibility complex (MHC) Class I. It is widely expressed on the surface of various cell types, including thymocytes, lymphocytes, granulocytes, monocytes, platelets, epithelial cells, and endothelial cells, though it is notably absent on erythrocytes. Due to its diminutive size, B2M is freely filtered by the kidney's glomerular membrane; however, typically less than 1% is excreted, as the majority undergoes efficient reabsorption in the proximal tubules. Its presence in biological fluids serves as a dynamic indicator of cell membrane renovation and overall cellular turnover, increasingly recognized as a valuable inflammatory biomarker in numerous inflammatory, hematologic, infectious, autoimmune, and neoplastic central nervous system (CNS) disorders.
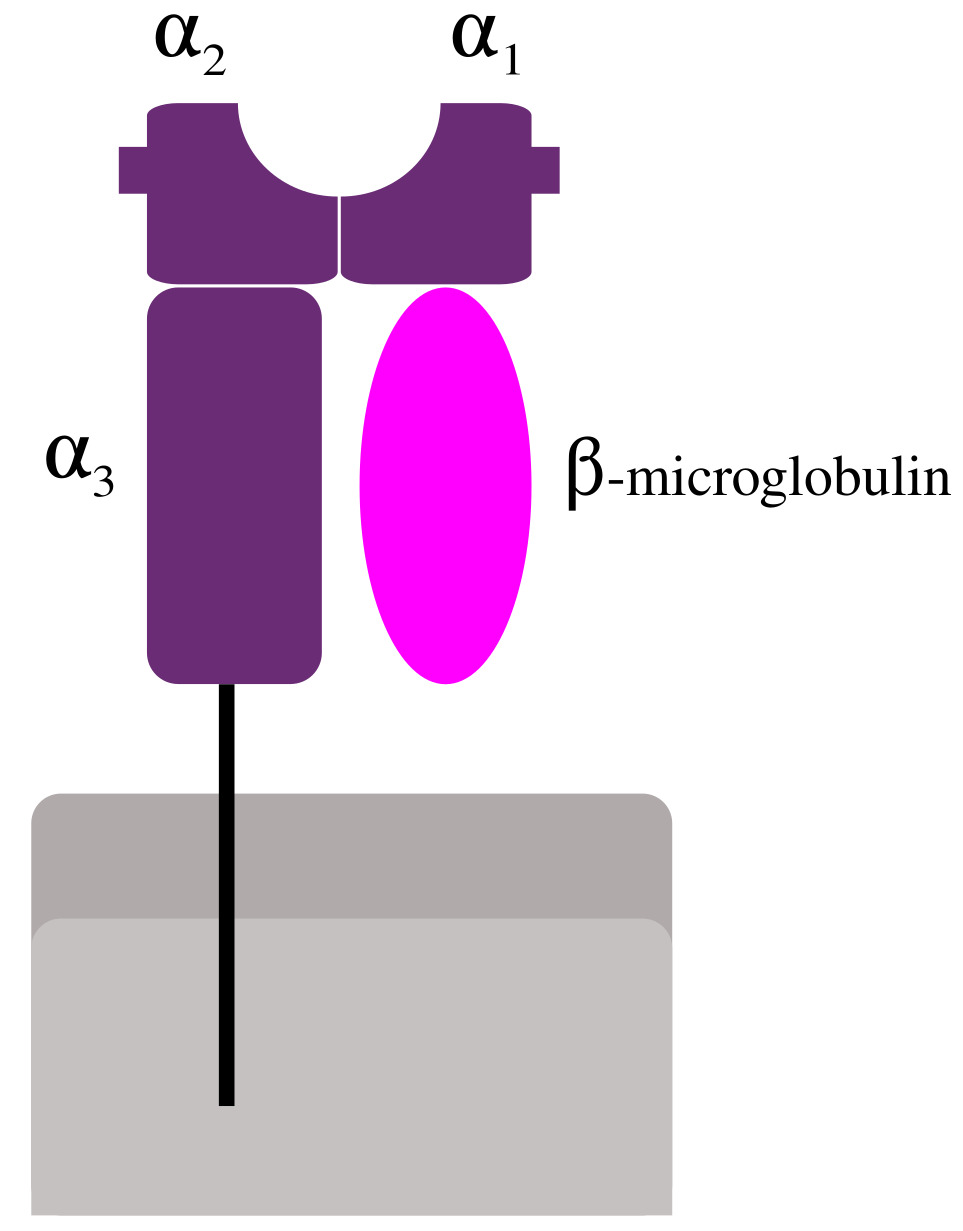 Fig.1 B2M is a component of MHC class I.Distributed under CC BY 2.5, from Wiki, without modification.
Fig.1 B2M is a component of MHC class I.Distributed under CC BY 2.5, from Wiki, without modification.
B2M Analysis Services at Creative Biolabs
Creative Biolabs offers comprehensive B2M analysis services, ensuring precise and reliable quantification across diverse sample types. Our advanced technical platforms encompass a variety of testing methods designed to meet your specific research needs. We expertly employ validated assays such as Immunoturbidimetry, highly sensitive Sandwich ELISA (enzyme-linked immunosorbent assay), and robust chemiluminescent immunometric assay, providing both singleplex and multiplex data options.
Taking latex-based immunoassay, for instance, a sample is diluted and reacted with a buffer that contains latex particles coated with an antibody specific for B2M. The formation of the antibody-antigen complex during the reaction results in an increase in turbidity, the extent of which is measured as the amount of light adsorbed at 545 nm. The B2M concentration in a sample is determined by constructing a standard curve from the absorbance of a reagent blank and a single-level calibrator.
A commercial human B2M sandwich ELISA kit is also frequently used for B2M quantification. The human B2M sandwich ELISA is designed to measure the amount of the target bound between a matched antibody pair. A B2M-specific antibody has been pre-coated in the wells of the supplied microplate. Samples, standards, as well as controls, are then added to these wells and bound to the immobilized capture antibody. The sandwich is formed by the addition of another B2M detection antibody, and a substrate solution is added that reacts with the enzyme-antibody-B2M complex to produce a measurable signal. The intensity of this signal is directly proportional to the concentration of B2M present in the original specimen.
 Fig.2 B2M analysis summary.
Fig.2 B2M analysis summary.
Service Workflow
Our B2M analysis service workflow is meticulously designed to ensure clarity, efficiency, and accurate results from initial contact to final delivery.
Your service begins with a detailed discussion with our expert team to thoroughly understand your specific research objectives, sample types, and desired analytical outcomes. This ensures our approach is precisely tailored to your project.
Following the consultation, you will securely send your biological fluid samples to our state-of-the-art laboratory. Clear instructions for sample collection and shipping will be provided to maintain sample integrity.
Upon receipt, your samples undergo rigorous quality control and appropriate preparation steps, including necessary dilutions or pre-treatments. Our skilled technicians then perform the selected B2M analysis assay—immunoturbidimetry, sandwich ELISA, or chemiluminescent immunometric assay—following strict, validated protocols.
Raw data generated from the assays are meticulously processed and subjected to stringent quality control checks. This involves statistical analysis, curve fitting (for quantitative assays), and verification against established performance criteria to ensure accuracy and reliability.
Once data analysis is complete and quality assured, you will receive a comprehensive report detailing your B2M concentrations, methodologically validated results, and relevant interpretations. Our team remains available for post-delivery consultation to address any further questions or assist with data integration into your broader research.
Applications
Renal Function Monitoring
Hematological Malignancy Prognosis
In hematologic oncology, B2M is a powerful prognostic marker, particularly for multiple myeloma and lymphoproliferative disorders. Its levels correlate with tumor burden and disease activity, aiding in staging, monitoring treatment response, and predicting patient outcomes.
Inflammatory and Autoimmune Disease Insight
Elevated B2M levels often signal significant immune activation and chronic inflammation. This makes B2M analysis relevant for assessing disease activity in conditions such as Systemic Lupus Erythematosus (SLE), particularly in cases with CNS involvement, and reflecting immune status in infectious diseases like HIV.
Service Highlights
- Precision Quantification: We deliver highly accurate and precise B2M quantification using advanced analytical platforms, including Immunoturbidimetry and Sandwich ELISA, ensuring data reliability for critical research decisions.
- Comprehensive Methodologies: Our breadth of technical platforms supports diverse research needs, offering both singleplex and multiplex B2M data options, from customized assay development to efficient, off-the-shelf solutions.
- Expert Interpretation: Beyond data generation, our team of seasoned biologists provides expert consultation and interpretation of B2M results, helping you integrate findings seamlessly into your drug development program or academic research.
FAQs
-
How do you ensure the accuracy of B2M analysis results?
We ensure the accuracy of B2M analysis results through rigorous adherence to validated protocols, implementation of stringent quality control measures, and the use of high-precision analytical instrumentation. Each assay run includes multiple quality controls and calibrators, providing confidence in the reliability and reproducibility of your data.
-
Can your B2M analysis differentiate between increased production and impaired clearance as causes for elevated levels?
Our B2M analysis provides quantitative measurements that, when interpreted in conjunction with other clinical parameters and patient history, can help distinguish between increased production and impaired clearance. Consulting with our expert team can provide guidance on interpreting these complex diagnostic scenarios.
-
Is B2M analysis offered as a standalone service, or must it be part of a larger panel?
B2M analysis at Creative Biolabs is available both as a standalone service for focused investigations and as part of broader biomarker panels. This flexibility allows you to select the most appropriate testing strategy for your specific research or clinical requirements.
-
What quality control measures are in place for the B2M analysis assays?
For B2M analysis, Creative Biolabs employs comprehensive quality control measures, including daily instrument calibration, regular use of internal and external quality control materials, and participation in proficiency testing programs. These steps ensure consistent assay performance and data integrity.
-
Are there any specific sample collection or storage requirements for B2M analysis?
Yes, specific sample collection and storage requirements are crucial for accurate B2M analysis, and detailed guidelines will be provided by Creative Biolabs upon service inquiry. Adherence to these protocols is essential to maintain sample integrity and ensure reliable analytical outcomes.
-
Do you offer customized B2M assay development for unique research needs?
Creative Biolabs specializes in offering customized B2M assay development to meet unique research needs, including adaptation to novel sample types or integration into complex experimental designs. Our team collaborates closely with clients to tailor solutions that align precisely with their specific objectives.
-
How does Creative Biolabs handle high-throughput B2M analysis requests?
Creative Biolabs is equipped to efficiently handle high-throughput B2M analysis requests through advanced automation platforms and streamlined workflows. This capability ensures that large volumes of samples can be processed rapidly and accurately, supporting large-scale research projects.
Whether you are envisioning a potential project or improving your current operations related to your portfolio, we can help you with innovative B2M bulk reagents. Our biomarker analysis service is furnished for academic research. If you want to order this test, please feel free to contact us for more information.
For Research Use Only.