Chimeric antigen receptor (CAR) T-cell therapy represents one of the most significant breakthroughs in cancer treatment over the past decade. This revolutionary form of immunotherapy has fundamentally transformed our approach to treating certain types of cancer, particularly hematological malignancies. By engineering a patient's own T cells to recognize and attack cancer cells, CAR-T therapy essentially creates a "living drug" that can provide long-lasting protection against cancer recurrence. Unlike traditional cancer treatments such as chemotherapy or radiation, which can damage both healthy and cancerous cells, CAR-T cells are precisely designed to target specific cancer antigens, offering a more targeted and potentially less toxic approach to cancer treatment.
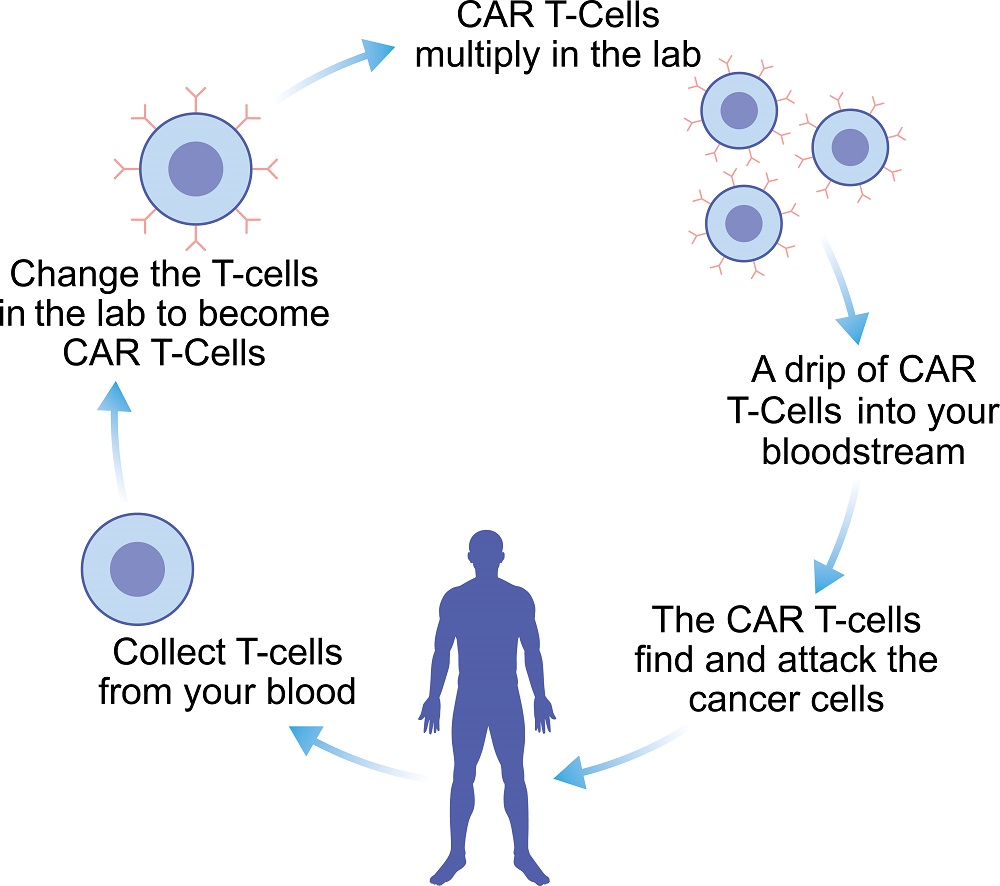 Fig.1 Overall processes of CAR T-cell therapy.
Fig.1 Overall processes of CAR T-cell therapy.
While initially developed for blood cancers such as leukemia and lymphoma, researchers and clinicians have been steadily expanding its applications, offering hope to patients with various forms of cancer who have exhausted conventional treatment options. The remarkable success of CAR-T therapy in treating previously intractable cases has spurred intensive research into adapting this approach for solid tumors and other challenging cancer types, potentially revolutionizing the future of cancer treatment.
The journey of CAR-T cell therapy from laboratory concept to approved treatment has been remarkable. Currently, several CAR-T cell products have received approval from regulatory authorities worldwide, including the FDA (Food and Drug Administration, USA), EMA (European Medicines Agency, European Union), NMPA (National Medical Products Administration, China), MHLW (Ministry of Health, Labour and Welfare, Japan), and other agencies. These approvals represent carefully validated therapeutic approaches that have demonstrated significant clinical benefits in specific patient populations.
Here is a comprehensive overview of globally approved CAR-T cell therapies:
Table 1 Approved CAR-T cell therapies worldwide
| Generic Name | Brand Name | Indication | Target | Region | Approval Date |
| Tisagenlecleucel | Kymriah | B-cell precursor ALL (patients ≤25 years) | CD19 | United States | August 2017 |
| European Union | August 2018 | ||||
| China | June 2023 | ||||
| Relapsed/refractory large B-cell lymphoma | United States | May 2018 | |||
| European Union | August 2018 | ||||
| China | June 2023 | ||||
| Relapsed/refractory follicular lymphoma | United States | May 2022 | |||
| European Union | June 2022 | ||||
| Axicabtagene ciloleucel | Yikaida | Large B-cell lymphoma | CD19 | China | June 2023 |
| Yescarta | United States | October 2017 | |||
| European Union | August 2018 | ||||
| Japan | January 2021 | ||||
| Follicular lymphoma | United States | March 2021 | |||
| European Union | June 2022 | ||||
| Second-line DLBCL | United States | April 2022 | |||
| European Union | August 2022 | ||||
| Brexucabtagene autoleucel | Tecartus | Mantle cell lymphoma | CD19 | United States | July 2020 |
| European Union | December 2020 | ||||
| B-cell precursor ALL (adults) | United States | October 2021 | |||
| European Union | January 2022 | ||||
| Lisocabtagene maraleucel | Breyanzi | Large B-cell lymphoma | CD19 | United States | February 2021 |
| European Union | April 2022 | ||||
| Second-line DLBCL | United States | June 2022 | |||
| European Union | November 2022 | ||||
| Idecabtagene vicleucel | Abecma | Multiple myeloma | BCMA | United States | March 2021 |
| European Union | August 2021 | ||||
| Ciltacabtagene autoleucel | Carvykti | Multiple myeloma | BCMA | United States | February 2022 |
| European Union | May 2022 | ||||
| Relma-cel | Carteyva | Large B-cell lymphoma | CD19 | China | September 2021 |
| Follicular lymphoma | China | February 2023 | |||
| Zevor-cel | Zercepic | Multiple myeloma | BCMA | China | June 2023 |
Tisagenlecleucel marked the beginning of the CAR-T era as the first approved CAR-T therapy worldwide. This CD19-directed genetically modified autologous T cell therapy has shown remarkable efficacy in both pediatric and young adult patients with B-cell acute lymphoblastic leukemia (B-ALL) and adult patients with various B-cell lymphomas. In the pivotal ELIANA trial for pediatric ALL, it achieved a complete remission rate of 83% within three months of infusion. For diffuse large B-cell lymphoma (DLBCL) patients in the JULIET trial, the overall response rate was 52%, with 40% of patients achieving complete remission. The therapy has demonstrated durable responses, with many complete remissions lasting over a year.
This CD19-directed CAR-T therapy has shown particularly impressive results in aggressive B-cell lymphomas. In the landmark ZUMA-1 trial, it achieved an overall response rate of 83% and a complete response rate of 58% in patients with refractory large B-cell lymphoma. The therapy is notable for its rapid manufacturing process (typical 17-day turnaround) and consistent product quality. The ZUMA-7 trial later demonstrated its superiority over standard care in second-line treatment of DLBCL, leading to its approval in earlier lines of therapy. Its approval in the US, EU, and Japan has established it as a standard treatment option in these regions.
As the locally manufactured version of axicabtagene ciloleucel in China, Yikaida has demonstrated comparable efficacy to Yescarta in clinical trials. The pivotal studies in Chinese patients with relapsed/refractory large B-cell lymphoma showed similar response rates to the global ZUMA trials. Local manufacturing capabilities help reduce the time from leukapheresis to infusion and improve accessibility for patients in China. The therapy received NMPA approval based on robust clinical data showing its safety and efficacy in the Chinese patient population.
Specifically designed for mantle cell lymphoma (MCL) and adult B-ALL, this therapy incorporates an additional manufacturing step to remove circulating malignant cells before T cell enrichment. In the ZUMA-2 trial for MCL, it achieved an impressive 93% response rate with 67% complete responses. The therapy has shown particular efficacy in patients who have failed Bruton's tyrosine kinase (BTK) inhibitor therapy, addressing a significant unmet need in this patient population.
Distinguished by its defined composition of CD4+ and CD8+ CAR-T cells, Breyanzi has demonstrated a favorable safety profile while maintaining high efficacy. In the TRANSCEND NHL 001 trial, it achieved a 73% overall response rate with 53% complete responses in heavily pretreated DLBCL patients. The defined composition of T cells appears to contribute to reduced toxicity compared to other CD19-directed therapies, with lower rates of severe cytokine release syndrome.
As the first B-cell maturation antigen (BCMA)-targeted CAR-T therapy approved for multiple myeloma, Abecma opened new possibilities for patients with relapsed/refractory disease. The KarMMa trial showed an overall response rate of 73% and complete response rate of 33% in heavily pretreated patients who had received at least three prior therapies. The therapy demonstrated meaningful improvements in quality of life and progression-free survival compared to standard care options.
This next-generation BCMA-targeted therapy features a dual epitope-binding design that may contribute to its high efficacy. In the CARTITUDE-1 study, it achieved an unprecedented 98% overall response rate with 78% stringent complete responses in heavily pretreated multiple myeloma patients. The depth and durability of responses have set new standards for CAR-T therapy in multiple myeloma.
Developed in China, Relma-cel has demonstrated comparable efficacy to other CD19-directed therapies while potentially offering improved accessibility in the Asian market. In pivotal trials, it showed an overall response rate of 75.9% in patients with relapsed/refractory large B-cell lymphoma with a manageable safety profile. The therapy has expanded treatment options for lymphoma patients in China and contributed to the growth of cellular therapy capabilities in the region.
As one of the newest approved BCMA-targeted therapies, Zevor-cel has shown promising results in Chinese patients with relapsed/refractory multiple myeloma. Clinical trials demonstrated high response rates comparable to other BCMA CAR-T products, with the advantage of local manufacturing capabilities that may improve accessibility for patients in China. In the CARTIFAN-1 study, zevor-cel demonstrated an overall response rate of 89.8% and a stringent complete response rate of 76.3%, with 95.2% of patients achieving minimal residual disease negativity. The therapy features an optimized manufacturing process with shortened vein-to-vein time, making it particularly suitable for the Chinese healthcare system.
While approved indications focus primarily on hematological malignancies, ongoing clinical trials are exploring CAR-T cell therapy's potential in a much broader range of cancers. These studies represent the cutting edge of cellular immunotherapy and are pushing the boundaries of what's possible in cancer treatment.
CAR-T cell therapy has emerged as a promising immunotherapeutic approach for glioblastoma (GBM), with several key targets showing potential in clinical development. The most extensively studied target is IL13Rα2, which is expressed in over 75% of GBM tissue with minimal expression in normal brain tissue, and clinical trials have shown encouraging results including one patient achieving a 7.5-month regression period. EGFRvIII, present in approximately 45% of GBM patients, has been evaluated in multiple trials showing safety but limited clinical benefit, partly due to antigen loss and adaptive resistance. HER2-targeted CAR-T therapy, despite initial safety concerns in early studies, has demonstrated promising results in recent trials with manageable toxicity profiles and evidence of clinical responses in some patients.
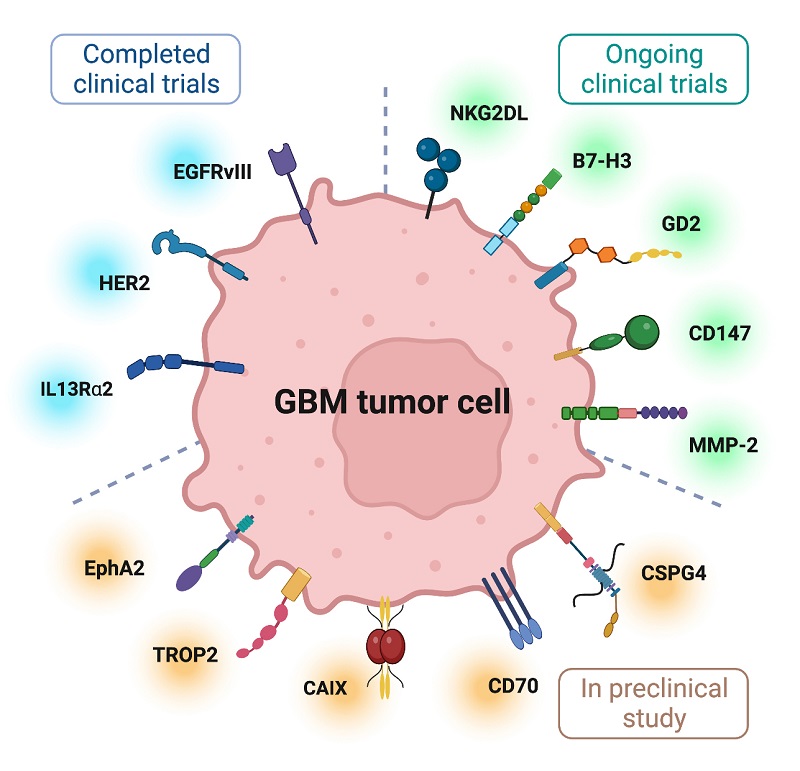 Fig.2 Several targetable tumor-associated antigens for GBM CAR T cell therapy1,5.
Fig.2 Several targetable tumor-associated antigens for GBM CAR T cell therapy1,5.
The field continues to expand with several novel targets entering clinical investigation. NKG2D ligands, frequently upregulated in GBM stem cells, have shown potent anti-tumor activity in preclinical models. B7-H3, expressed in over 70% of GBM samples, is being evaluated in multiple ongoing trials with encouraging preliminary results. Other emerging targets include GD2, which has shown particular promise in H3K27M-mutant diffuse midline gliomas, CD147, which has demonstrated selective targeting capabilities, and chlorotoxin-directed CAR-T cells targeting MMP-2. These newer approaches are complemented by innovative engineering strategies like synthetic notch (synNotch) CARs for improved control and specificity, as well as the development of alternative cell therapies such as CAR NK and CAR macrophage therapies, which have shown unique advantages in preclinical studies and early phase trials.
CAR-T cell therapy has emerged as a promising immunotherapeutic approach for treating pancreatic cancer, with several potential targets being investigated. The key targets include mesothelin (expressed in 80-85% of pancreatic cancers), CD133, prostate stem cell antigen (PSCA), Claudin 18.2, HER2, MUC1, and carcinoembryonic antigen (CEA). Clinical trials have shown encouraging results, particularly with mesothelin-targeted CAR-T cells demonstrating superior efficacy in non-metastatic and locally advanced cases. HER2-targeted CAR-T therapy has also shown promise in phase I trials, achieving partial remission in some patients.
However, significant challenges remain in implementing CAR-T therapy for pancreatic cancer. The primary obstacles include the immunosuppressive tumor microenvironment, limited T cell infiltration, and physical barriers created by dense stromal tissue. Additionally, the therapy faces challenges such as "on-target, off-tumor" effects and cytokine release syndrome. Current research focuses on combining CAR-T therapy with other treatment modalities, developing strategies to overcome the immunosuppressive microenvironment, and enhancing CAR-T cell persistence and efficacy. The development of next-generation CAR-T cells with improved targeting and reduced toxicity remains an active area of investigation.
CAR-T cell therapy shows promise for ovarian cancer treatment, with multiple targets under investigation including ERBB2/HER2 (showing good preclinical results), mesothelin (MSLN, expressed in 30% of cases), MUC16/CA125 (expressed in >80% of cases), and others like EPCAM, FOLR1, and CD24. Clinical trials have demonstrated safety and some efficacy, though challenges remain. A notable case showed tumor reduction using αPDCD1/MESO-CAR-T cells, with metastatic nodule diameter decreasing from 71.3mm to 39.1mm after 2 months.
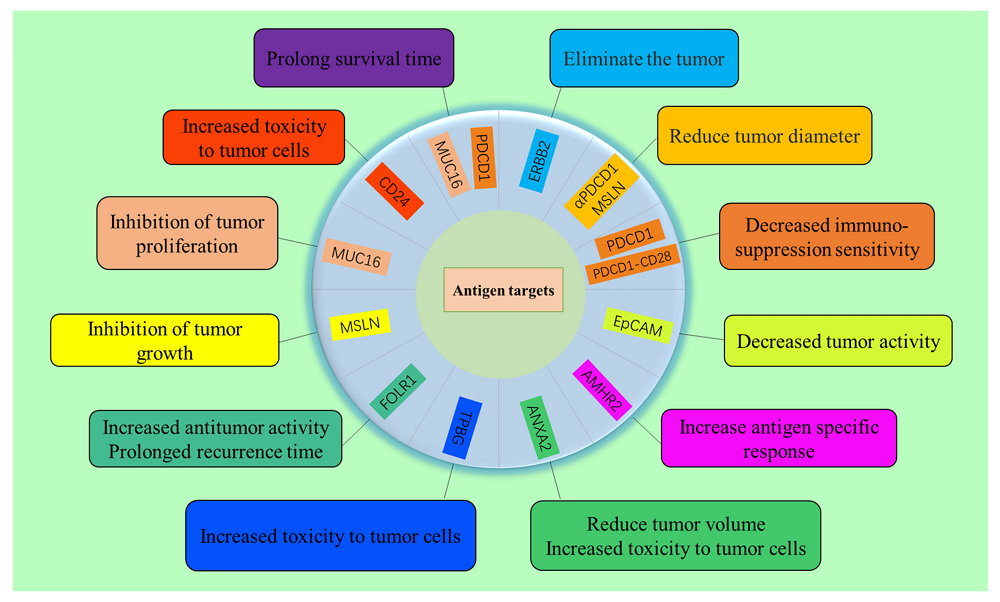 Fig.3 Antigenic targets of OC cells and related functions of CAR-T cells targeting these tumor antigens2,5.
Fig.3 Antigenic targets of OC cells and related functions of CAR-T cells targeting these tumor antigens2,5.
Recent developments focus on overcoming key challenges through multiple strategies: engineering armored CAR-T cells to secrete proinflammatory cytokines, improving CAR-T cell persistence and tumor infiltration, developing multi-antigen targeting approaches, and combining with other therapies like checkpoint inhibitors, chemotherapy, or radiation. Novel approaches include using dual-specific CARs, tandem CARs, and various targeting strategies to enhance efficacy while reducing toxicity. The tumor microenvironment remains a major challenge, with ongoing work to address immunosuppression, hypoxia, and metabolic barriers.
CAR-T cell therapy has shown promising potential for treating lung cancer, with several targets being actively investigated in both preclinical and clinical trials. The key targets include mesothelin (MSLN), EGFR, receptor tyrosine kinase-like orphan receptor 1 (ROR1), mucin-1 (MUC1), prostate stem cell antigen (PSCA), HER2, CEA, and PD-L1. Early studies have demonstrated encouraging results, particularly with MSLN-targeted CAR-T cells showing significant inhibitory effects on cancer cell proliferation and invasion. EGFR-targeted CAR-T cells have also exhibited potent cytotoxic activity against lung cancer cells in preclinical models.
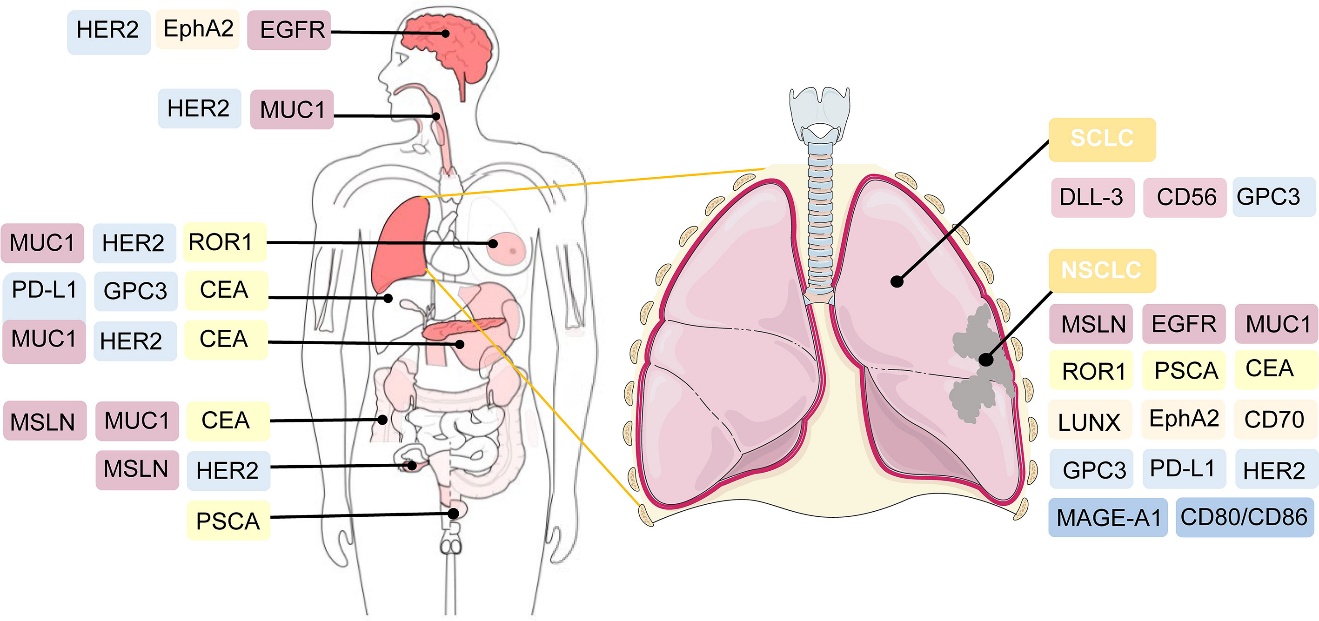 Fig.4 Potential targeted antigens for CAR-T cell therapy in lung cancer3,5.
Fig.4 Potential targeted antigens for CAR-T cell therapy in lung cancer3,5.
However, several challenges need to be addressed for successful implementation of CAR-T therapy in lung cancer. These include treatment-related toxicities like cytokine release syndrome, on-target off-tumor toxicity, physical barriers preventing T cell infiltration into tumor sites, and the immunosuppressive tumor microenvironment. Various engineering strategies are being developed to overcome these challenges, such as modifying CAR design to reduce toxicity, using dual-target CAR-T cells to prevent antigen escape, expressing chemokine receptors to enhance tumor infiltration, and incorporating mechanisms to overcome the immunosuppressive microenvironment. The advancement of these engineering solutions, particularly through gene editing technology, holds promise for improving the safety and efficacy of CAR-T therapy in lung cancer treatment.
CAR-T therapy has shown promise for treating T-cell malignancies, with CD7, CD5, CD30, and CD37 emerging as key targets. Clinical trials have demonstrated encouraging results, particularly with CD7-targeted CAR-T therapy achieving complete remission rates of 80-95% in relapsed/refractory T-cell acute lymphoblastic leukemia (T-ALL). However, several unique challenges exist, including T-cell fratricide (CAR-T cells destroying each other), T-cell aplasia (depletion of normal T-cells), and potential contamination of CAR-T products with malignant T-cells.
Recent innovations focus on overcoming these challenges through various approaches. These include genetic modifications to prevent fratricide, using single-domain antibody-derived or naturally selected CAR-T cells for improved efficacy, and developing allogeneic CAR-T products to avoid tumor contamination. Novel targets like TRBC1 and CCR9 are being explored to minimize T-cell aplasia. Additionally, combination strategies and dual-targeting approaches (like CD5/CD7 bispecific CAR-T cells) are being investigated to enhance therapeutic efficacy and prevent antigen escape.
CAR-T cell therapy has shown potential but faces unique challenges in treating Acute Myeloid Leukemia (AML). Several targets have been investigated in clinical trials, including CD123 (expressed in >75% of cases), CD33 (expressed in ~90% of cases), CLL-1, CD7, and NKG2D ligands. Clinical trials targeting CD123 have demonstrated some efficacy, with several patients achieving complete remission, while CD33-targeted therapy has shown modest responses. However, most responses tend to be transient, with many patients experiencing disease progression or relapse.
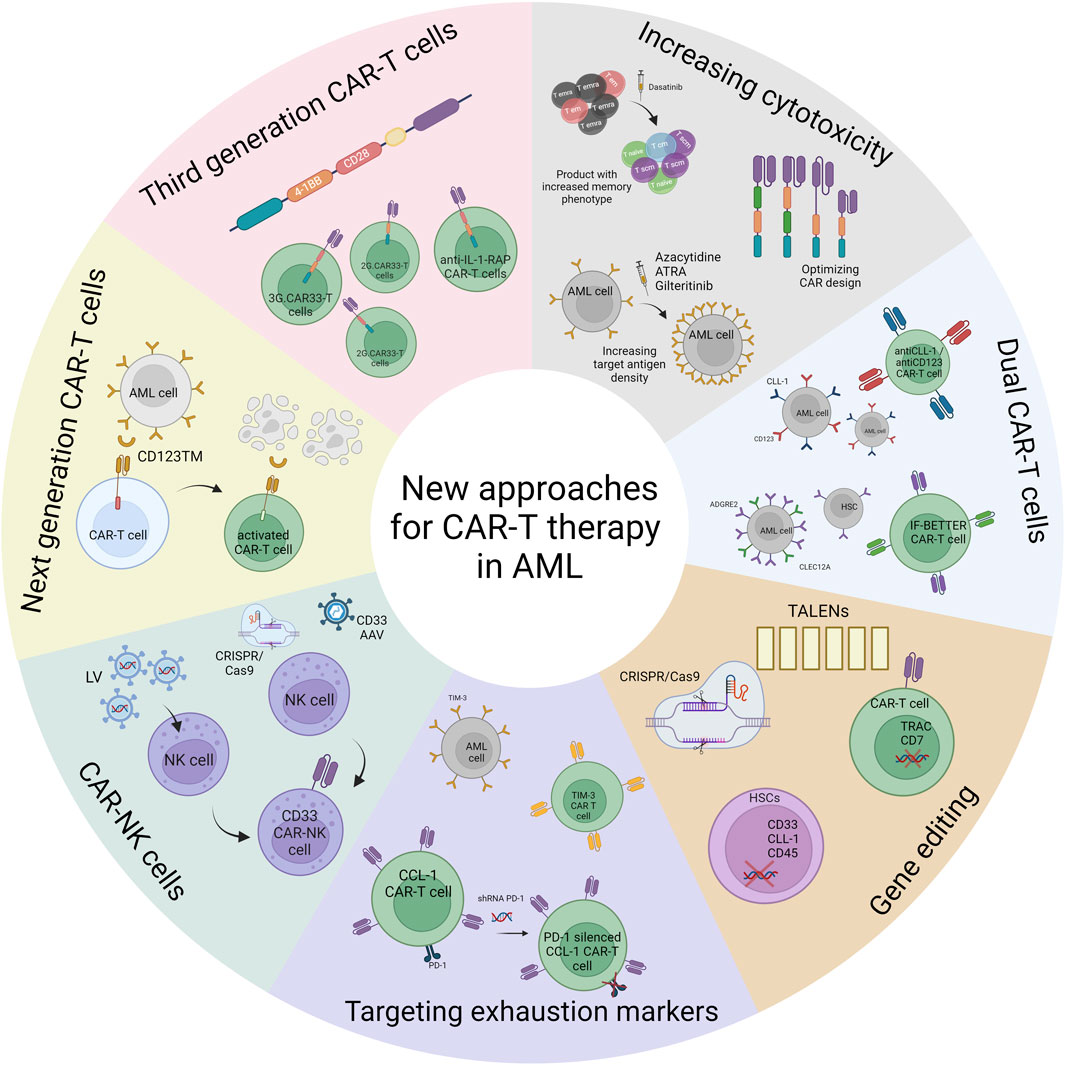 Fig.5 New approaches for CAR-T cell therapy in AML4,5.
Fig.5 New approaches for CAR-T cell therapy in AML4,5.
Recent innovations focus on next-generation CAR designs with targeting modules (like CD123TM), dual-targeting strategies to prevent antigen escape, and engineering CAR-T cells to express chemokine receptors for better tumor infiltration. Additionally, gene editing tools are being utilized to create allogeneic CAR-T cells from healthy donors and to knockout target antigens from hematopoietic stem cells before transplantation to prevent off-target toxicities. The field is also exploring CAR-NK cells as an alternative approach, which may offer advantages such as reduced toxicity and no risk of graft-versus-host disease.
The future of CAR-T cell therapy holds exciting possibilities for treating an even broader range of cancers. Several innovative approaches are being developed that could significantly expand the therapeutic landscape:
Multi-targeted CAR-T cells represent a promising direction for preventing antigen escape, a common mechanism of resistance to current CAR-T therapies. These "smart" CAR-T cells can recognize multiple tumor antigens simultaneously or switch targets based on the tumor's expression profile. Early research suggests this approach could be particularly valuable in treating heterogeneous solid tumors and reducing the risk of relapse.
Allogeneic CAR-T cells, or "off-the-shelf" products, are being developed to overcome the manufacturing challenges and time constraints associated with autologous treatments. These universal CAR-T cells could potentially be manufactured in advance and stored until needed, making the therapy more accessible and reducing costs. Early clinical trials are exploring their safety and efficacy in various cancer types.
Novel target antigens are being investigated for various cancer types:
Combination approaches are also being explored, integrating CAR-T cell therapy with other treatment modalities:
The development of CAR-T cells with enhanced functionality is another exciting area of research. These next-generation products might include:
While significant challenges remain, particularly in treating solid tumors, the rapid pace of innovation in CAR-T cell therapy suggests that we may see dramatic expansions in its therapeutic applications in the coming years. As our understanding of cancer biology and cellular engineering continues to advance, CAR-T cell therapy is likely to become an increasingly important tool in the fight against cancer, potentially offering hope to patients with previously untreatable malignancies.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION