Liposomes have been at the forefront of nanotechnology-driven drug delivery systems for decades, revolutionizing biomedical applications due to their flexibility, biocompatibility, and ability to deliver a wide range of molecules. Creative Biolabs, with years of experience in the industry, offers cutting-edge, customized liposome development services. Our conventional liposome development Service provides advanced solutions tailored to meet the unique needs of drug delivery applications, leveraging the expertise of our liposome specialists.
Conventional liposomes are spherical vesicles composed of phospholipid bilayers, often including cholesterol to enhance stability. These lipid vesicles encapsulate an aqueous core, allowing them to carry both hydrophilic and hydrophobic drugs. First introduced as a drug delivery system in the 1980s, conventional liposomes provide significant advantages by improving drug biodistribution and pharmacokinetics, while minimizing systemic toxicity. They have proven highly effective in encapsulating chemotherapeutic agents, antimicrobial drugs, and vaccines.
The structure of conventional liposomes consists of a bilayer of phospholipids (such as phosphatidylcholine or sphingomyelin) that can interact with both hydrophobic and hydrophilic drugs. The lipophilic drugs are incorporated into the bilayer, while hydrophilic drugs are contained within the aqueous center.
Key components of conventional liposomes include:
Conventional liposomes are particularly appealing because of their biocompatibility, flexibility in drug loading, and ability to target specific cells through passive mechanisms, particularly in diseases involving the mononuclear phagocyte system (MPS).
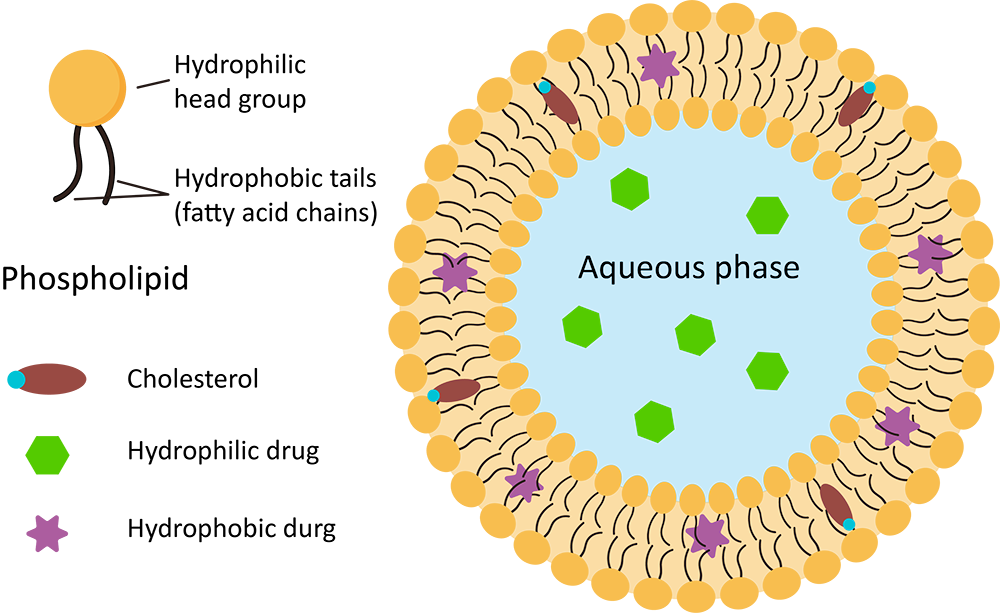 Fig.1 Structure of conventional liposome
Fig.1 Structure of conventional liposome
Conventional liposomes have become a versatile tool in various therapeutic research fields, thanks to their unique ability to encapsulate and deliver drugs effectively. These applications are not only scientifically significant but also offer tangible advantages in both medical and consumer products. Here's how conventional liposomes are being utilized across different domains:
| Application | Description | Key Benefits |
| Cancer | Liposomes deliver agents like doxorubicin directly to tumor cells. | Enhances agent concentration in target cells, minimizes impact on healthy tissues, leverages the enhanced permeability and retention (EPR) effect. |
| Antimicrobial and Antifungal | Liposomal formulations, such as amphotericin B, target specific cells like macrophages. | Reduces compound toxicity (e.g., nephrotoxicity) while ensuring efficient delivery to target cells. |
| Vaccine Development | Liposomes act as adjuvants, facilitating antigen delivery to immune cells and controlling release. | Supports stronger immune response, enables controlled release of antigens, and ensures long-lasting protection in vaccines. |
| Targeting the Mononuclear Phagocyte System (MPS) | Liposomes are designed to interact with macrophages in areas where certain pathogens are localized. | Provides precise delivery of agents to the MPS, where specific conditions are focused, improving the specificity of applications. |
| Cosmetics and Nutritional Supplements | Liposomes enhance the absorption of vitamins and antioxidants in skincare and nutritional products. | Improves bioavailability and absorption of active ingredients, enhancing the performance of cosmetic and dietary products. |
At Creative Biolabs, we understand that each research and drug development project comes with its specific needs. Conventional liposomes, the first-generation nanocarriers, remain one of the most effective and widely used systems in the market. Our services are designed to enhance drug delivery efficacy, reduce toxicity, and improve pharmacokinetics. We offer fully customizable services that allow precise control over lipid composition, vesicle size, surface charge, and encapsulation efficiency to suit your exact requirements. We provide tailored solutions for every stage of liposome development, ensuring that each formulation is optimized for the client's specific application.
| Service | Description |
| Consultation & Advisory | In-depth consultation to understand client needs and provide tailored solutions for liposome design. |
| Liposome Formulation Design | Customized lipid composition design for optimal encapsulation and delivery based on project needs. |
| Drug Encapsulation | High-efficiency drug encapsulation into liposomes, preserving the integrity and efficacy of the drug. |
| Liposome Purification | Advanced purification techniques to ensure the stability and homogeneity of liposome preparations. |
| Formulation Characterization | Comprehensive analysis of liposome properties: size, stability, zeta potential, and encapsulation efficiency. |
| Freeze-Drying (Lyophilization) | Lyophilization services to improve shelf-life and long-term storage of liposomal products. |
| Production Scaling & Optimization | Seamless transition from laboratory-scale production to large-scale manufacturing. |
| Final Report | Detailed report including formulation design, preparation processes, and product characterization. |
At Creative Biolabs, we specialize in the development of high-performance conventional liposomes tailored to diverse pharmaceutical and cosmetic applications. Utilizing advanced formulation techniques and stringent quality control, we ensure reproducibility, stability, and functionality in every batch.
Case 1: Anionic Liposomes (Cholesterol, DOPC, DOPS)
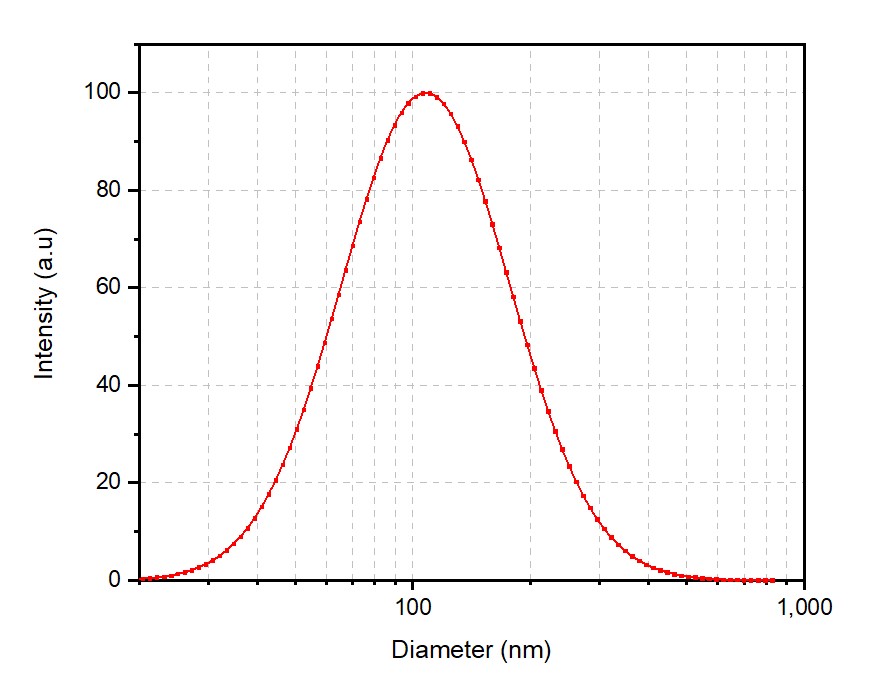
The low PDI and consistent particle size reflect our precision in formulation and process control.
Case 2: Neutral Liposomes (Cholesterol, DOPC, DPPC)

The nearly neutral zeta potential minimizes non-specific interactions, enhancing biocompatibility.
With extensive experience in conventional liposome development, we guarantee consistent quality and performance. Our services offer flexible delivery formats, including ready-to-use liquid formulations or complimentary lyophilization (freeze-drying) to significantly enhance product stability and shelf life. Trust us to deliver the optimal liposome solution for your needs—precise, reliable, and tailored to excellence.
Creative Biolabs utilizes advanced platforms to deliver precise, efficient, and reproducible conventional liposome development services. Here are the three key platforms that drive our innovative approach to liposome formulation:
In addition to these core platforms, we also employ zeta potential measurement, lyophilization technology, and scale-up facilities to ensure optimal stability, storage, and manufacturing scalability for liposome products. These supporting platforms guarantee that our liposomal formulations are robust, reproducible, and ready for clinical or research applications.
Building on years of research, Creative Biolabs continuously optimizes its conventional liposome services to meet your research demands. With enhanced drug stability, improved delivery precision, and versatile applications across pharmaceuticals, gene therapy, and vaccines, our solutions offer tailored formulations that align with the specific needs of each project. Additionally, our production processes are designed to be fast, customizable, and scalable. We control liposome size and properties through precise instrument parameters, ensuring that the final product meets client expectations while maintaining reproducible and contamination-free conditions.
Our advantages in conventional liposome formulation development include:
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseServices
Online Inquiry