At Creative Biolabs, we believe the potential of any therapeutic is only as strong as its delivery vehicle. In the dynamic field of lipid-based drug delivery systems, Neutral Liposomes offer a safe, reliable, and versatile platform. These non-ionic carriers are crucial for maximizing systemic stability and achieving effective drug exposure. Our dedicated Neutral Liposome Development Service provides end-to-end expertise, transforming complex APIs into optimized formulations.
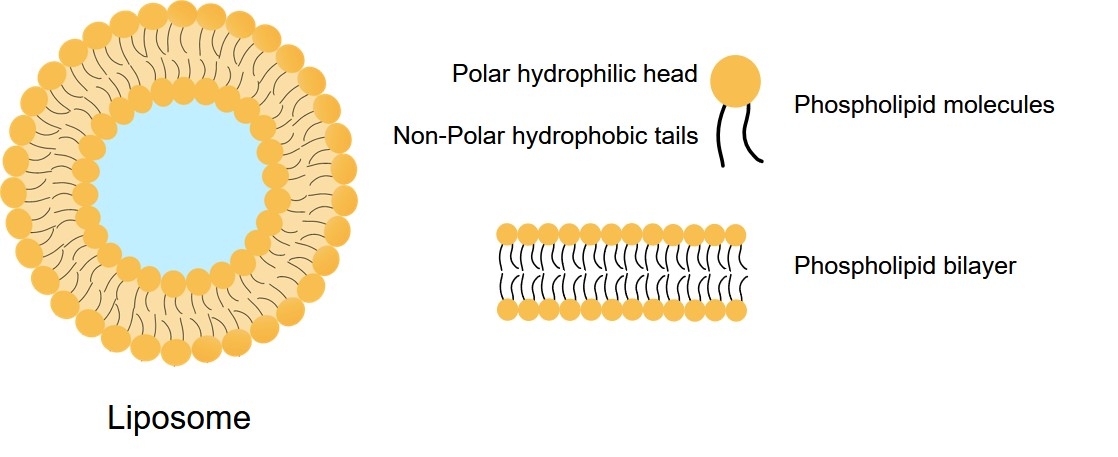
Neutral liposomes are defined by their surface charge, or lack thereof. Unlike highly charged cationic or anionic liposomes, they are composed primarily of neutral, naturally occurring phospholipids (such as HSPC or DSPC) and Cholesterol. This non-ionic character is critical for their function, as it minimizes non-specific electrostatic interactions with proteins and cell membranes in vivo. This fundamentally reduces systemic side effects and cytotoxicity, making them the preferred platform for long-circulating, systemic drug delivery applications.
The performance of a neutral liposome is highly dependent on the synergistic properties of its constituent lipids. Formulations typically combine structural phospholipids that dictate membrane rigidity and helper lipids like cholesterol that influence fluidity, packing density, and stability. Selecting the correct combination is paramount for successful drug encapsulation and in vivo function.
| Neutral Lipid | Role in Formulation | Key Benefit |
|---|---|---|
| HSPC (Hydrogenated Soy PC) | Structural Phospholipid | Confers excellent membrane rigidity and physical stability. |
| DSPC | Structural Phospholipid | Provides maximum chemical and physical stability for long circulation. |
| Cholesterol (Chol) | Helper Lipid | Stabilizes the bilayer, reduces leakage, and modulates membrane fluidity. |
| DOPE | Helper Lipid | Promotes membrane destabilization for enhanced endosomal release. |
To ensure longevity in the bloodstream, our neutral liposomes are sterically stabilized—a process often achieved through the incorporation of polyethylene glycol (PEG) onto the liposome surface (PEGylation).
This process yields two profound benefits, which are core to their success:
Ready to enhance your therapeutic potential?
Creative Biolabs offers a full-spectrum, turnkey approach designed to take your active pharmaceutical ingredient (API) from concept to a viable, scalable formulation. Our services are anchored in scientific rigor and operational excellence.
We initiate every project with a thorough characterization of your drug cargo (small molecule, peptide, or imaging agent) to select the optimal lipid composition and loading technique. This includes detailed Lipid Screening and precise determination of the necessary molar ratio optimization.
We develop high-efficiency loading protocols (including remote loading and active loading strategies) to maximize drug loading capacity and entrapment efficiency while maintaining batch consistency through controlled extrusion and homogenization.
We overcome the inherent instability of aqueous liposome dispersions by employing state-of-the-art lyophilization protocols. Using optimized lyoprotectants, we generate highly stable, proliposome powders that guarantee extended shelf life and maintain crucial physicochemical properties upon reconstitution.
The final step focuses on tuning the product for its intended biological use and confirming all critical quality attributes (CQAs), including the final zeta potential (confirming neutral charge), particle size, PDI, and encapsulation efficiency.
Neutral liposomes are essential, versatile carriers for sensitive compounds, proven across crucial fields of advanced research:
Our reputation as a reliable, innovative, and client-centric partner is built on three pillars of professional excellence:
Creative Biolabs is dedicated to advancing the field of lipid-based drug delivery systems. Our Neutral Liposome Development Service is your definitive path to achieving stable, safe, and highly effective therapeutic carriers. To move your research forward, contact our dedicated scientific team today. We are ready to discuss your API's unique characteristics and tailor a formulation strategy that meets your specific research goals.
| Product Name | Description | Inquiry |
|---|---|---|
| Fluorescent Liposome (Neutral) | Neutral liposomes pre-labeled with a non-leaking fluorescent dye for imaging and cellular uptake studies. | Inquiry |
| DSPC:Chol Liposomes | Classic neutral liposomes, often used as a structural backbone in doxorubicin-loaded formulations. | Inquiry |
| DSPC Liposomes | Plain, saturated neutral liposomes for basic encapsulation and membrane fusion studies. | Inquiry |
| DOPE:Chol Liposomes | Neutral/fusogenic formulation for enhanced destabilization and potential co-delivery applications. | Inquiry |
| DOPE Liposomes | Non-bilayer forming lipid liposomes for studies involving membrane curvature and destabilization. | Inquiry |
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseServices
Online Inquiry