In the rapidly advancing field of lipid-based drug delivery systems, the demand for carriers that offer both high loading efficiency and superior biocompatibility is paramount. While cationic lipids have traditionally dominated nucleic acid delivery, their toxicity profile often limits clinical translation. PG-based anionic liposomes have emerged as a sophisticated alternative, utilizing divalent cation bridging to encapsulate payloads while mimicking endogenous cellular components for enhanced safety. At Creative Biolabs, we leverage decades of formulation expertise to design and manufacture precision-engineered anionic liposomes, positioning us as your premier partner for overcoming the complex barriers of modern therapeutic delivery.
Phosphatidylglycerol (PG) is a naturally occurring anionic phospholipid found abundantly in pulmonary surfactant and mitochondrial membranes. Unlike neutral carriers, PG-based liposomes possess a negative surface charge derived from the phosphate group in the glycerol head. This charge provides electrostatic repulsion between vesicles, preventing aggregation and ensuring exceptional long-term colloidal stability compared to zwitterionic formulations.
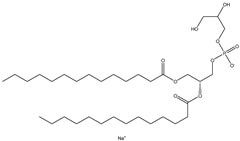 Fig. 1 The structural schematic diagram of PG.1
Fig. 1 The structural schematic diagram of PG.1
The unique biological identity of PG-based liposomes facilitates specific interactions within the body:
Researchers are increasingly turning to anionic systems to mitigate the adverse effects of cationic lipids, such as hemolysis and complement activation. By utilizing divalent cations (e.g., Ca²⁺, Mg²⁺) to bridge the negative lipid headgroups with negatively charged nucleic acids, PG-liposomes achieve high encapsulation efficiency without the cytotoxicity associated with permanent positive charges.
Creative Biolabs provides a holistic development platform designed to take your therapeutic concept from the bench to pre-clinical reality. We do not simply manufacture; we engineer solutions tailored to your specific biological targets.
| Step | Description | Starting Materials | Deliverables | Estimated Time |
|---|---|---|---|---|
| 1 | Consultation & Design |
Project Requirements Target Payload |
Lipid selection strategy Proposal generation |
< 1 Week |
| 2 | Lipid Screening & Prep | Synthetic Lipids (DOPG, etc.), Payload | Preparation of lipid films/powders and optimization of molar ratios. | 2-4 Weeks |
| 3 | Formulation & Sizing |
Lipid Film Buffer Payload |
Thin-film hydration followed by extrusion or microfluidics to achieve target size. | |
| 4 | Purification & Loading | Crude Liposomes | Removal of unencapsulated drug via dialysis or column chromatography. | |
| 5 | Quality Control (QC) | Purified Liposomes | Comprehensive analysis (DLS, Zeta, HPLC). | 1 Weeks |
| 6 | Delivery | / | Product and COA | < 1 Week |
PG-based liposomes act as potent self-adjuvants. By targeting Antigen-Presenting Cells (APCs) via scavenger receptors, they enhance the presentation of encapsulated antigens, eliciting robust humoral and cellular immune responses without the need for toxic additives.
Overcoming the toxicity limits of cationic lipids, our PG-based formulations utilize calcium-mediated bridging to condense nucleic acids. This provides a safer, non-immunogenic vehicle for the systemic administration of gene therapies and mRNA vaccines.
The unique interaction between anionic lipids and the skin's stratum corneum enhances the penetration of dermatological actives. PG liposomes are excellent candidates for topical treatments of inflammatory skin conditions like psoriasis and eczema.
Creative Biolabs stands at the forefront of lipid technology, offering sophisticated PG-based Anionic Liposome Development services that bridge the gap between concept and cure. Our commitment to scientific rigor, combined with our state-of-the-art manufacturing capabilities, ensures that your drug delivery challenges are met with precise, effective solutions. Do not let formulation challenges stall your therapeutic development. Partner with us to leverage the safety and specificity of anionic liposomes.
| Product Name | Description | Inquiry |
|---|---|---|
| DOPG | 1,2-dioleoyl-sn-glycero-3-phospho-(1'-rac-glycerol), >99% purity synthetic anionic lipid. | Inquiry |
| DOPG:Chol Liposome | Stabilized anionic liposome formulation with cholesterol for enhanced membrane rigidity. | Inquiry |
| Ready-to-Use Anionic Liposomes | Pre-formed empty PG liposomes for control experiments or passive loading. | Inquiry |
Reference
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseServices
Online Inquiry