Anionic Liposome Development Services
Background Our Services Workflow Applications Why Choose Us Related Services & Products FAQs
At Creative Biolabs, we understand that the success of a next-generation therapeutic relies not only on the active pharmaceutical ingredient (API) but, crucially, on its delivery vehicle. As specialists in Lipid-based Drug Delivery Systems, we focus on providing scalable, high-quality solutions that overcome in vivo challenges. Our Anionic Liposome Development Service offers researchers and pharmaceutical developers a superior, low-toxicity platform for encapsulating a wide range of therapeutic and diagnostic agents.
Understanding Anionic Liposomes Technology
What Are Anionic Liposomes?
Anionic liposomes are lipid vesicles characterized by a net negative surface charge (Zeta potential) arising from the inclusion of negatively charged lipids such as Phosphatidylglycerol (PG) or Phosphatidylserine (PS). This structural characteristic allows them to efficiently encapsulate both hydrophilic compounds in their aqueous core and hydrophobic molecules within the lipid bilayer, making them exceptionally versatile drug carriers for both small molecules and complex biopharmaceuticals.
Why Anionic Liposomes are Superior in Safety and Stability
Unlike conventional cationic liposomes, which possess a positive charge that leads to higher, often dose-limiting, cytotoxicity and non-specific binding to blood components, anionic liposomes offer a clear safety advantage. The native negative charge minimizes non-specific interaction with negatively charged plasma proteins and cell surfaces. This inherent Reduced Cytotoxicity and electrostatic repulsion contribute to superior stability and prolonged circulation times in vivo, significantly improving the therapeutic index.
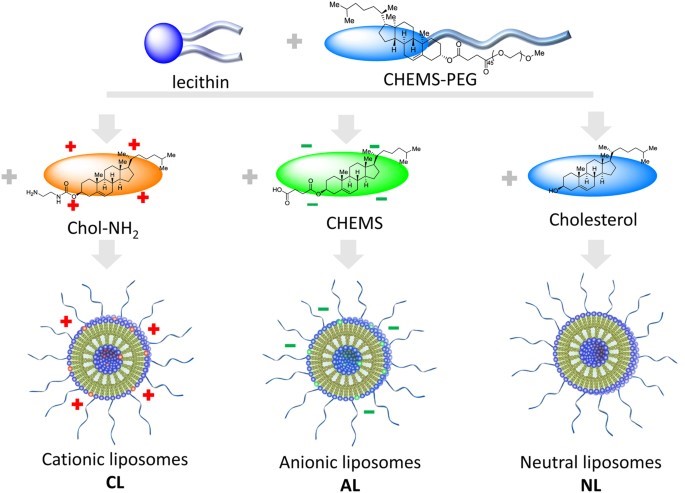 Fig. 1 Anionic, cationic and neutral liposomes formulation. 1
Fig. 1 Anionic, cationic and neutral liposomes formulation. 1
Key Components of High-Performance Anionic Formulations
Creative Biolabs meticulously controls the molecular architecture of every liposome. Key components used in our anionic formulations include:
-
Anionic Lipids: PG, PS for precise Zeta potential control.
-
Structural Lipids: Phosphatidylcholines (e.g., DSPC, DOPC) and Cholesterol (Chol) to stabilize the membrane and optimize fluidity.
-
Steric Stabilizers: PEGylated lipids (e.g., DSPE-PEG2000) for Steric Stabilization, which creates a protective, hydrophilic layer that shields the liposome from opsonization and clearance, resulting in Long-Circulating properties.
Comprehensive Anionic Liposomes Development Services
As your dedicated partner in lipid-based drug delivery, Creative Biolabs integrate our deep expertise in lipid chemistry with scalable processes to guarantee high batch-to-batch consistency and performance.
We develop bespoke lipid compositions and molar ratios, specializing in efficient and stable encapsulation of your specific Active Pharmaceutical Ingredient (API), whether small molecule, peptide, or nucleic acid.
Rigorous testing includes precise measurement of particle size, PDI, and Zeta potential. We provide comprehensive data necessary for regulatory submissions and performance validation.
Ensuring your formulation is scalable, we optimize manufacturing processes (e.g., extrusion, microfluidics) for high yield, reproducibility, and production.
Lyophilization & Long-Term Stability Studies
We design and validate freeze-drying cycles with optimized Lyoprotectants to ensure exceptional long-term stability and a simplified logistics profile for your final product.
Workflow
Applications of Anionic Liposomes in Modern Research
The unique combination of stability, low immunogenicity, and high payload capacity makes our anionic liposomes ideal non-viral vectors for high-impact applications:
-
Cancer Therapy: Delivering chemotherapeutic agents (e.g., Doxorubicin, Paclitaxel) with reduced systemic toxicity and enhanced accumulation at tumor sites via controlled circulation.
-
Gene/Nucleic Acid Delivery: Serving as low-immunogenic carriers for DNA, RNA, miRNA, or siRNA, especially in environments where reduced positive charge interaction is desirable.
-
Vaccine Development & Immunotherapy: Acting as carriers for antigens or adjuvants, leveraging controlled release to potentiate and guide the desired immune response.
-
Diagnostic Imaging: Encapsulation of contrast agents or fluorescent probes for targeted in vivo tracking and advanced diagnostics.
Why Choose Creative Biolabs for Anionic Liposomes Development?
Our commitment to scientific excellence and client partnership distinguishes us in the field of lipid-based drug delivery:
-
Low-Toxicity Focus: We specialize in anionic systems, specifically engineered to minimize cytotoxicity and non-specific immune activation, enhancing your therapeutic's safety profile.
-
Advanced Lyophilization Expertise: Our patented techniques guarantee the long-term stability and convenience of Lyophilized Liposomes, simplifying shipping and storage logistics.
-
Tailored Lipid Chemistry: We offer bespoke liposome-like structural control, precisely modulating charge, size, and composition for optimal performance with your specific API.
-
Accelerated R&D: Our integrated workflow and rapid-turnaround characterization services are designed to Accelerate R&D timelines and reduce development costs.
Creative Biolabs is committed to delivering state-of-the-art Anionic Liposome Development Services that enhance the safety and effectiveness of your therapeutic programs. By specializing in low-toxicity, long-circulating, and shelf-stable liposomal systems. Ready to advance your drug delivery project? Partner with our expert team to design a customized anionic liposome formulation today.
Related Services & Products
Related Services
Related Products
|
Product Name
|
Description
|
Inquiry
|
|
DOPG
|
High-purity anionic lipid widely used for creating stable, low-toxicity liposomes.
|
Inquiry
|
|
DSPS
|
Anionic lipid that aids in specific cellular recognition and is often used in diagnostic applications.
|
Inquiry
|
|
DSPE-PEG Lipid
|
Steric stabilizer for manufacturing long-circulating (PEGylated) liposomes.
|
Inquiry
|
FAQs
What is the primary difference between anionic and cationic liposomes?
Cationic liposomes carry a positive charge and are often used for nucleic acid delivery due to electrostatic attraction, but they carry a higher risk of cytotoxicity. Anionic liposomes carry a negative charge, offering reduced toxicity and enhanced in vivo stability and circulation time.
What is the significance of Zeta potential and PDI in liposome development?
Zeta potential is a measure of the surface charge, critical for predicting colloidal stability and interaction with cell membranes. PDI (Polydispersity Index) measures the uniformity of particle sizes; a low PDI is essential for reproducible in vivo performance and is a key regulatory metric.
Can you encapsulate hydrophobic drugs in an anionic liposome?
Yes. Liposomes are amphiphilic. Hydrophobic drugs partition into the lipid bilayer, while hydrophilic drugs are sequestered in the aqueous core. Anionic composition does not preclude efficient hydrophobic drug loading.
Why is lyophilization (freeze-drying) necessary for my liposome formulation?
Lyophilization converts the liquid suspension into a dry powder, dramatically improving long-term stability and extending shelf life from months to years. It also simplifies shipping without requiring cold-chain logistics, and our process ensures easy reconstitution upon hydration.
Reference
-
Yin, Qinqin, et al. "Effects of liposomes charge on extending sciatic nerve blockade of N-ethyl bromide of lidocaine in rats." Scientific reports 6.1 (2016): 38582. https://doi.org/10.1038/srep38582. Distributed under Open Access license CC BY 4.0, without modification.

For Research Use Only. Not For Clinical Use
 Fig. 1 Anionic, cationic and neutral liposomes formulation. 1
Fig. 1 Anionic, cationic and neutral liposomes formulation. 1
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical Use