Enzymatic Deglycosylation
Overview of Enzymatic Deglycosylation
Glycoproteins can be divided into N-linked glycan chains and O-linked glycan chains by types of glycopeptide chains in glycoproteins. In enzymatic deglycosylation, different glycosidase enzymes should be selected according to the types of glycopeptide chains. For N-linked glycopeptides, the commonly used enzymes are peptide N-glycosidase A (PNGase A), peptide N-glycosidase F (PNGase F), endoglycosidase F (Endo F), and endoglycosidase H (Endo H). Both PNGase A and PNGase F can hydrolyze the GlcNAc-Asn bond. PNGase F enzyme removes high mannose, complex, and hybrid glycan chains, but cannot remove fucose glycan chains. PNGase A can remove fucose-type glycan chains, so these two enzymes are often used in combination to achieve complete deglycosylation. Endo F and Endo H cleave the glycosidic bond between the two adjacent GlcNAc in the N-glycan chain pentasaccharide core Manα1-6 (Manα1-3) Manβ1-4GlcNAcβ1-4GlcNAc, release the peptide containing one GlcNAc. They also remove high mannose type, compound glycan chain, two-antenna, and three-antenna structures. The above endoglycosidase cannot directly act on O-linked glycopeptides. For O-linked glycopeptides, a series of exoglycosidase is first required to release oligosaccharides from the non-reducing end of the glycan chain, such as β-N -Acetyl glucosaminidase, sialidase, etc. are first hydrolyzed one by one. Only protein core 1 (Gal β1-3 GalNAC-Thr/Ser) and core 3 (GlcNAC β1-3 GalNAC-Thr/Ser) are left, and then use O-glycosidase to act on the GalNAc-Ser/Thr bond.
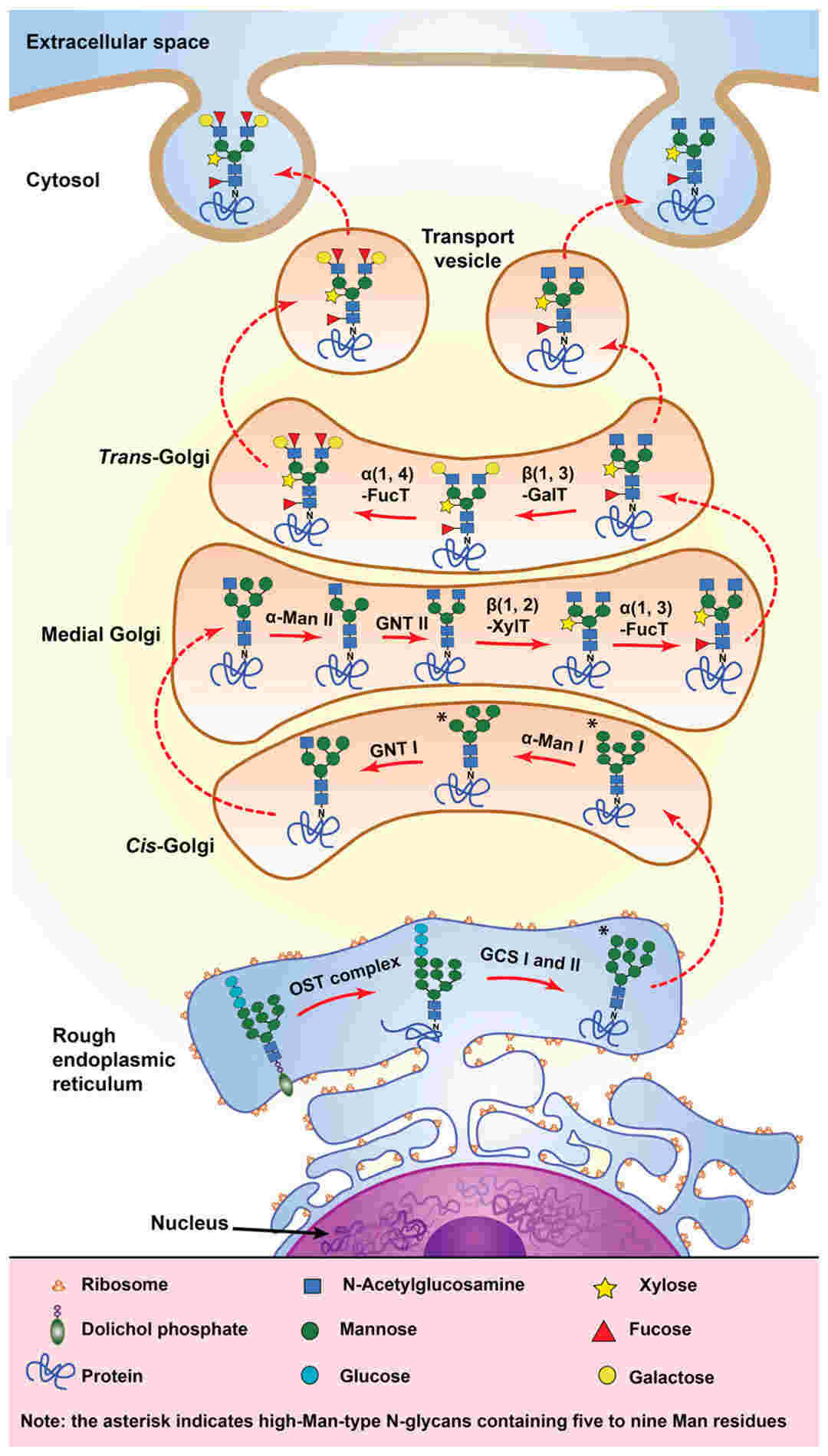 Fig.1 N-glycosylation pathway in plant cells.1, 2
Fig.1 N-glycosylation pathway in plant cells.1, 2
Enzymatic Deglycosylation Strategies
-
Endoglycosidase deglycosylation
The endoglycosidase includes PNGase F, PNGase A, Endo F, Endo H, and O-glycosidase. These enzymes have different specificities for glycoproteins and are currently the most widely used enzymes in deglycosylation engineering and can be applied to many fields of biology. PNGase F can hydrolyze almost all N-linked glycans in glycoproteins. PNGase A can effectively remove fucose-type glycoproteins. Endo F has weak protein specificity. Endo F1 can cleave high mannose and heterozygous glycoproteins in N-glycoprotein. Endo F2 and F3 can cleave two-antennary and three-antennary glycoproteins in N-glycoprotein. However, the Endo F3 enzyme does not work on hybrid and oligomannose glycoproteins. Fucosylated glycoprotein limits the enzyme activity of Endo F2 but promotes the activity of the Endo F3 enzyme. Endo H can cleave the chitobiose core structure of oligomannose in N-glycoprotein and some hybrid oligosaccharides. O-glycosidase, or endo-α -n-acetylgalactosamine, catalyzes the removal of O-linked disaccharide units from glycoprotein core 1(Galβ1-3 Galnac-Thr /Ser) and core 3(GlcNACβ1-3 Galnac-Thr /Ser).
-
Exoglycosidase deglycosylation
Exoglycosidase is the earliest type of enzyme that acts on the glycan chain of glycoproteins, for example, sialidase, β-galactosidase, β-N-acetylglucosaminidase, α-L-fructosidase, α-N-acetylgalactosidase, α-mannosidase, β- Mannosidase, etc. Through the combined action of these enzymes, the glycan groups of glycoproteins can be specifically released. Different degrees of deglycosylation correspond to different functional activities. In the deglycosylation process of erythropoietin, when the terminal glycan such as galactose, sialic acid, and acetylglucosamine are removed by the specific exonuclease, its activity gradually increases, and further cutting of the internal glycan chain will result in loss of activity. Sialidase and β-galactosidase can slowly hydrolyze sialic acid residues and galactose residues in glycoproteins, respectively. α-Mannosidase is a highly specific exonuclease: α1-2,3mannosidase catalyzes the hydrolysis of oligosaccharide α1-2 and α1-3-D-mannose residues; α1-6 mannose glycosidase catalyzes unbranched α1-6 glycosidic bonds of oligosaccharides. When two enzymes are used in combination, α1-6 mannosidase can catalyze branched α1-6 glycosidic bonds.
-
Mixed enzymes to remove glycosyls
Due to the heterogeneity of glycans, especially in the presence of sialic acid glycosylation or bisecting N-acetylglucosamine, a single enzyme deglycosylation cannot achieve complete removal of glycan groups. Thus the mixed-use of multiple enzymes is required.
Services at Creative Biolabs
Enzymatic deglycosylation is one the wildest used methods in the exploration of glycosylation. With a strong foundation and extensive experience, Creative Biolabs provides comprehensive glycoprotein analysis services to help our customers accelerate their research:
If you are focusing on glycoprotein research or you have any questions about our services, please don't hesitate to contact us for more information.
References
-
Ghahremani, Mina, Kyla A. Stigter, and William Plaxton. "Extraction and characterization of extracellular proteins and their post-translational modifications from Arabidopsis thaliana suspension cell cultures and seedlings: a critical review." Proteomes 4.3 (2016): 25.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 N-glycosylation pathway in plant cells.1, 2
Fig.1 N-glycosylation pathway in plant cells.1, 2

