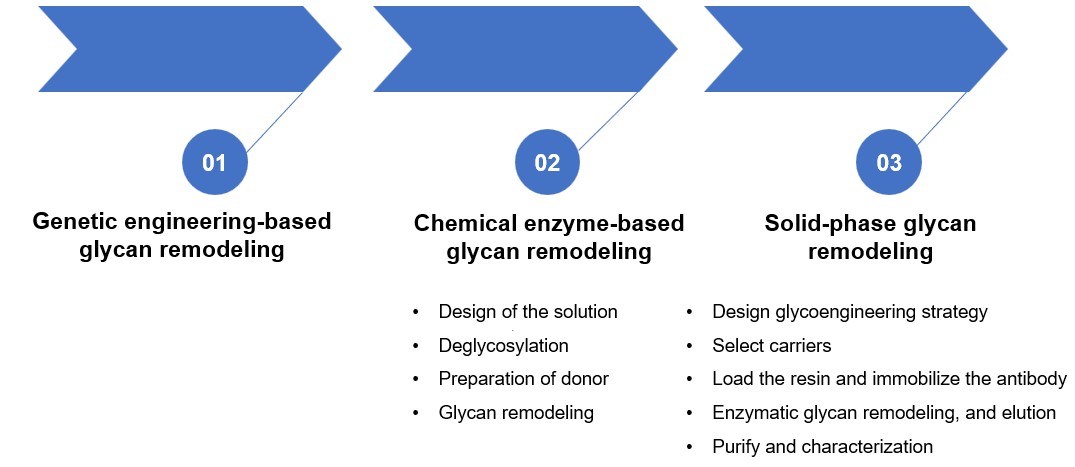
Glycan Remodeling Service
Various Glycan Remodeling Services at Creative Biolabs
Therapeutic antibodies are biological agents that modulate immune responses or directly intervene in disease processes by targeting specific biomolecules or cell surface receptors, which have important clinical applications and are widely developed as an important biopharmaceutical. Therapeutic antibody carries some heterogeneous glycans on their own protein structure, which affects the stability, immunogenicity, and efficacy of the antibody. Therefore, it is extremely important to perform glycoengineering-based glycan remodeling for therapeutic antibodies. Creative Biolabs has established a comprehensive and professional Glycoengineering Development Platform, and we provide a variety of strategies to support glyco-chemistry-based glycan remodeling.
-
Strategy 1: Genetic engineering-based glycan remodeling service
We synthesize specific glycosyl structures and introduce the glycosyl into the antibody structure through gene editing or transgenic technology, while combining specific glycoengineering enzymes to achieve the remodeling of glycans in organisms.
-
Strategy 2: Chemical enzyme-based glycan remodeling service
-
Design of the solution: Different enzymes have different selectivities and reaction conditions for glycosyl groups. We select appropriate chemical enzymes to achieve glycan remodeling according to client needs and antibody characteristics.
-
Deglycosylation: We enzymatically deglycosylate antibodies using endoglycosidases.
-
Preparation of donor: We use chemical methods to synthesize the glycosyl derivatives that need to be remodeled as donors.
-
Glycan remodeling: The glycosyl donor is installed on the antibody protein under the catalysis of glycosynthase, thereby achieving glycan remodeling.
The method improves the ability to adjust protein glycosylation. The use of chemical enzymes for glycan remodeling can achieve precise glycosyl modification, providing support for the optimization of antibody drugs and personalized treatment. For example, we use α-2,6-sialyltransferase to sialylate IgG to generate the glycan composition of G2S1F and G2S2F, and use β-1,4-galactosyltransferase to galactosylate IgG to generate high levels of G2F glycans. In addition, we provide a variety of high-quality glycan-related products to assist and accelerate the in vitro glycosylation process of antibodies.
Table 1 List of glycan-related products we provide.
|
Endoglycosidase
|
Glycosyltransferase
|
Related products
|
|
Endo-A
|
α-2,3-Sialyltransferase
|
Sugar modifying enzyme
|
|
Endo-M
|
α-2,6-Sialyltransferase
|
Activated glycosyl donor
|
|
Endo-S
|
β-1,4 Galactosyltransferase
|
Antibody Fc glycan remodeling kit
|
|
Endo-S/S2
|
Recombinant α-2,3-Sialyltransferase
|
Fc glycan conjugating ADC kit
|
|
Endo-S/S2 mutant
|
Recombinant α-2,6 Sialyltransferase
|
α-2,6-Sialyltransferase Residual Protein Kit
|
|
SpBgaA
|
Recombinant β-1,4 Galactosyltransferase
|
β-1,4-Galactosyltransferase Residual Protein Kit
|
-
Strategy 3: Solid-phase glycan remodeling (SPGR) service
In addition, the in vitro activities of more glycoengineering enzymes are gradually becoming known, which provides new development strategies for glycan remodeling. For example, endoglycosidase S and its mutants were used to synthesize glycans and then replaced natural IgG glycans. Based on the principle of solid-phase peptide synthesis, we use SPGR technology to perform antibody glycoengineering services.
-
First, we design an appropriate glycoengineering strategy according to the antibody that needs to be modified and the desired glycosyl structure.
-
We select appropriate solid support materials as carriers, such as resins, for chemical modification on their surfaces to achieve glycosyl remodeling.
-
Then, We load the resin, immobilize the antibody, and perform steps such as washing, enzymatic glycan remodeling, and elution for efficient glycan remodeling. Moreover, the two processes of washing and enzymatic glycan remodeling can be repeated, thereby exchanging reaction buffers and enzymes. The antibody as substrate remains immobilized on the resin until all glycan remodeling processes are completed.
-
Finally, we isolate and purify the reconstituted antibodies, fluorescently label them, and perform mass spectrometry characterization to ensure that the obtained product meets the requirements.
Published data
The diversity and inconsistency of N-glycosylation play a key role in the functions and biostability of therapeutic proteins. Recently, the emergence of in vitro glycan remodeling technology has made it possible to add customized oligosaccharides to recombinant proteins, thereby producing structurally uniform and customized glycoproteins. In this study, the authors demonstrated an efficient and simple chemoenzymatic glycan remodeling technology using a monoclonal antibody as an example. This technology used the expression system of Nicotiana benthamiana plants to combine in vitro chemoenzymatic glycosylation with in vivo deglycosylation to successfully prepare a homogeneous antibody glycoform with two terminal galactose residues and no fucose modification. This antibody showed enhanced functional activity. This method simplified the traditional glycan remodeling process and provided a cost-effective and scalable platform for producing antibodies with improved biological properties.
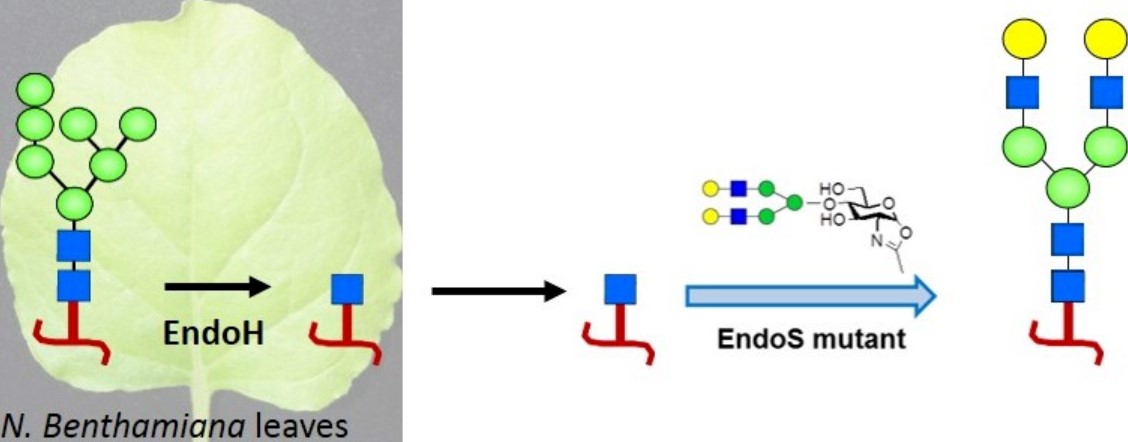 Fig.1 Plant expression system-based glycan remodeling scheme.1
Fig.1 Plant expression system-based glycan remodeling scheme.1
Advantages
-
Unique technology: We have mastered advanced glycosyl remodeling technology, which changes the glycosyl structure of antibodies and improves their efficacy and bioavailability.
-
Custom service: We design and implement personalized glycosylation remodeling programs according to client needs to meet the specific requirements of different antibody projects.
-
Efficient synthesis: We have an efficient glycosyl synthesis service, which can quickly synthesize complex glycosyl structures, and ensure the purity and stability of the synthesized products.
Applications
-
Drug development: Glycosyl remodeling technology improves the efficacy, stability, and immunogenicity of biopharmaceuticals, thereby improving their therapeutic effect.
-
Antibody engineering: Glycosyl remodeling can be used to change the glycosyl structure of the antibody, adjust the immunogenicity and activity of the antibody, and improve the bioavailability and efficacy of the antibody.
-
Cell surface engineering: Glycosyl remodeling technology changes the glycosyl structure on the cell surface and regulates the signaling, adhesion, and recognition properties of cells.
Creative Biolabs has experienced and professional Antibody Glycoengineering teams to provide glyco-chemistry-based glycan remodeling services to clients around the world. We use glycosyl remodeling technology to regulate the immunogenicity of antibodies and reduce the risk of immune reactions, thereby improving therapeutic effects. Please contact us if you are interested in our glycan remodeling service.
FAQ
Q1: How to choose a suitable glycan remodeling strategy?
A1: First, we will conduct a comprehensive assessment of the client's antibody properties and target glycosylation structure. Depending on the specific needs, we choose different glycan remodeling strategies. Each strategy has its unique advantages. The chemoenzymatic method can achieve more precise glycosylation, and the solid phase method has obvious advantages in repeatability and productivity.
Q2: How does the chemoenzymatic method ensure the accuracy of glycosylation?
A2: The accuracy of the chemoenzymatic method comes from the selection of highly specific glycosyltransferases and appropriate reaction conditions. We use a validated enzyme system and optimize reaction parameters to ensure that the target glycan can be located and firmly bound to the specified position. In addition, after the reaction, we will analyze the remodeled antibodies through mass spectrometry and other technologies to ensure that they meet the requirements.
Q3: How to ensure that the remodeled antibodies meet the quality requirements?
A3: We use a series of analytical methods to confirm the quality of the remodeled products, including liquid chromatography, mass spectrometry, fluorescent labeling, and other technologies to evaluate the purity, glycosylation type, and binding efficiency of the antibodies. All production steps and inspections meet strict quality control standards to ensure that each batch of products meets the specific requirements of clients.
Customer Review
Precise Glycan Remodeling
“The glycan remodeling service provided by Creative Biolabs was very precise. The remodeled antibodies performed well in the effect test, which fully demonstrated the advancedness and effectiveness of their technology.”
Personalized Glycan Remodeling Program
“In response to our specific needs, Creative Biolabs designed a completely personalized glycan remodeling program. The delivered antibodies were rigorously identified by mass spectrometry and the quality reached the highest standards, which fully met our requirements.”
Reference
-
Bennett, Lindsay D., et al. "Implementation of glycan remodeling to plant-made therapeutic antibodies." International Journal of Molecular Sciences 19.2 (2018): 421. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services


 Fig.1 Plant expression system-based glycan remodeling scheme.1
Fig.1 Plant expression system-based glycan remodeling scheme.1

