Precision Plant Hormone Analysis Solutions at Creative Biolabs
Plant hormones function as biochemical conductors, coordinating growth patterns and stress responses across botanical systems. Unlike animal counterparts produced in discrete glands, these signaling molecules are synthesized in various tissues with both localized and systemic effects. Accurate understanding of plant hormone levels is critical to optimizing crop yields, enhancing stress resistance, and promoting advances in plant biotechnology. Creative Biolabs provides comprehensive Plant Chemistry Profiling services, including Plant Pigment, Element, Vitamin, hormone, etc., which empower users to delve deeper into plant biology.
-
Plant Hormone Compound Analysis Service
Focusing on minute but potent phytohormones like ethylene and jasmonates, our liquid chromatography-mass spectrometry (LC-MS) platforms detect compounds active at picomolar concentrations. The service accommodates diverse analytes from classical auxins to modern brassinosteroids, capturing their dynamic interplay within plant systems.
Table 1 Plant hormone compounds detection list.
|
Category
|
Compound Name
|
Abbreviation
|
Compound Name
|
Abbreviation
|
|
Ethylene
|
Aminocyclopropane carboxylic acid
|
ACC
|
-
|
-
|
|
Salicylic acid
|
Salicylic acid
|
SA
|
-
|
-
|
|
Brassinolides
|
Brassinolide
|
BR
|
-
|
-
|
|
Abscisic acid
|
Abscisic acid
|
ABA
|
|
|
|
Cytokinins
|
Zeatin
|
-
|
Dihydrozeatin riboside
|
DHZR
|
|
Dihydrozeatin
|
-
|
N6-(delta2-Isopentenyl) adenine
|
2IP
|
|
Trans-zeatin-riboside
|
TZR
|
N6-(delta2-Isopentenyl) adenosine
|
IPA
|
|
Jasmonic acids
|
Methyl jasmonate
|
MeJA
|
Dihydrojasmonic acid
|
H2JA
|
|
Jasmonic acid
|
JA
|
Jasmonic acid-isoleucine
|
JA-Ile
|
|
Auxin
|
3-Indoleacetamide
|
IAM
|
3-Indolepropionic acid
|
IPA
|
|
3-Indolecarboxylic acid
|
ICA
|
3-Indolebutyric acid
|
IBA
|
|
Indole-3-acetic acid
|
IAA
|
3-Indoleacetonitrile
|
IAN
|
|
Gibberellins
|
Gibberellin A1
|
GA1
|
Gibberellin A4
|
GA4
|
|
Gibberellic A3
|
GA3
|
Gibberellin A7
|
GA7
|
-
Gibberellin Compound Analysis Service
Gibberellin compounds are a class of plant hormones. It is derived from tetracyclic diterpene skeletons, including more than 130 structural variants (such as GA3 and GA4). They promote stem elongation, seed germination, and fruit development by activating GID1 receptors to degrade DELLA proteins. Due to the significant functional differences of different gibberellin isomers and the complex metabolic dynamics in plants, we use specific LC-MS/MS analysis to detect various gibberellins.
Table 2 Gibberellin compounds detection list.
|
Category
|
Compound Name
|
Abbreviation
|
Compound Name
|
Abbreviation
|
|
Gibberellin (GA)
|
Gibberellin A1
|
GA1
|
Gibberellin A15
|
GA15
|
|
Gibberellin A3
|
GA3
|
Gibberellin A19
|
GA19
|
|
Gibberellin A4
|
GA4
|
Gibberellin A20
|
GA20
|
|
Gibberellin A5
|
GA5
|
Gibberellin A24
|
GA24
|
|
Gibberellin A6
|
GA6
|
Gibberellin A29
|
GA29
|
|
Gibberellin A7
|
GA7
|
Gibberellin A34
|
GA34
|
|
Gibberellin A8
|
GA8
|
Gibberellin A51
|
GA51
|
|
Gibberellin A9
|
GA9
|
Gibberellin A53
|
GA53
|
-
Brassinosteroid Compound Analysis Service
Brassinolide compounds are plant hormones with steroids as the skeleton, represented by brassinolide, which are multifunctional regulatory hormones with both growth promotion and stress resistance. Its content is extremely low, and slight structural differences can significantly affect its activity. We use highly sensitive LC-MS/MS or bromination-derived gas phase mass spectrometry (GC-MS) for accurate quantification. Individual analysis of this type of compound optimizes stress-resistance breeding strategies and provides key metabolic data for the development of drought-resistant and salt-alkali-resistant crops.
Table 3 Brassinolide compounds detection list.
|
Category
|
Compound Name
|
Abbreviation
|
Compound Name
|
Abbreviation
|
|
Brassinolide (BR)
|
Brassinolide
|
BL
|
28-Norcastasterone
|
28-NorCS
|
|
24-epi-Brassinolide
|
24-EpiBL
|
28-Homocastasterone
|
28-HomoCS
|
|
28-Norbrassinolide
|
28-NorBL
|
6-Deoxo-24-epi-Castasterone
|
6-Deoxo-24-epiCS
|
|
28-Homobrassinolide
|
28-HomoBL
|
Typhasterol
|
TY
|
|
Castasterone
|
CS
|
28-Norteasterone
|
28-NorTE
|
|
6-Deoxocastasterone
|
6-DeoxoCS
|
28-Norcastasterone
|
28-NorCS
|
Sample Requirements and Analysis Process
Creative Biolabs can analyze a wide range of plant samples. The analysis process typically involves sample preparation, chromatographic separation, MS detection, and identification analysis.
Creative Biolabs specializes in delivering precise phytohormone analysis solutions tailored to both academic and industrial requirements. Our methodology harnesses state-of-the-art analytical platforms to support groundbreaking discoveries in plant science. For protocol customization or project consultations, our technical team remains available for immediate discussion. Please contact us to customize your plant hormone analysis service.
Published Data
The authors developed an LC-MS/MS assay for accurate, reliable, and comparable measurement of key phytohormone levels in Arabidopsis thaliana. The experimental design quantified six critical regulators:
-
Auxin (IAA)
-
Stress hormones ABA and JA
-
Signaling molecules SA and OPDA
-
Jasmonate conjugate JA-Ile
The mass spectral analysis employed ion trap technology to achieve cross-laboratory reproducibility - a longstanding challenge in comparative phytohormone studies. This standardized protocol enables consistent data generation across varied experimental setups.
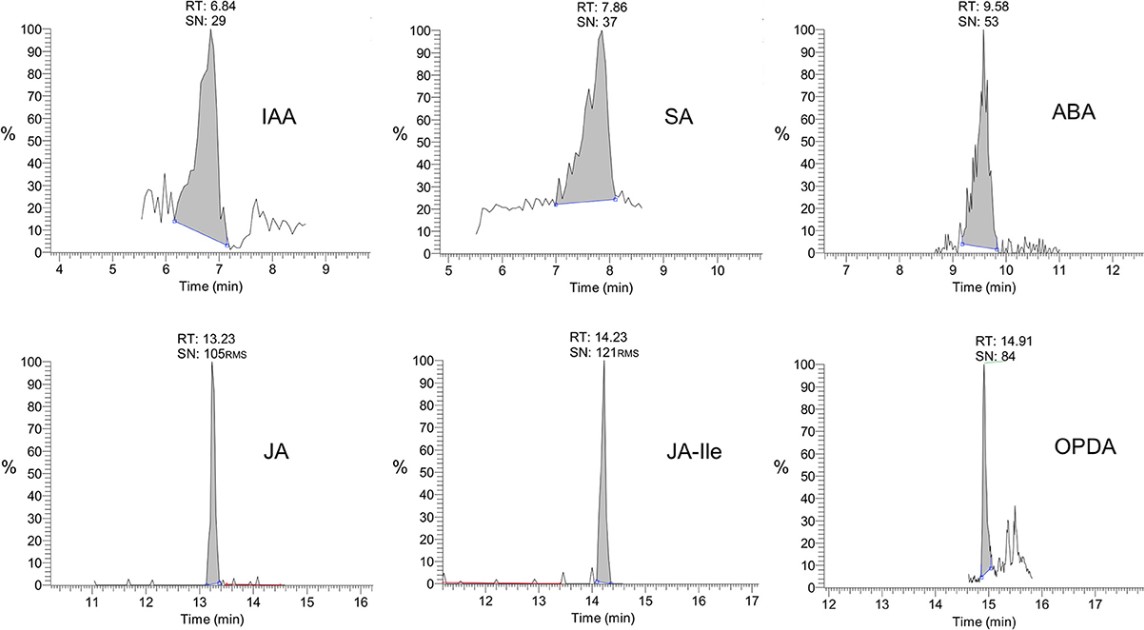 Fig.1 Selected reaction monitoring (SRM) chromatograms of phytohormones in samples.1
Fig.1 Selected reaction monitoring (SRM) chromatograms of phytohormones in samples.1
FAQs
Q1: What analytical methods do you use for hormone measurement?
A1: Our primary quantification approach employs LC-MS/MS technology, particularly effective when handling intricate biological samples due to its high-resolution capabilities. For specific high-volume screening needs, we implement ELISA platforms as an alternative. While LC-MS/MS excels in multi-hormone detection accuracy, ELISA becomes advantageous when prioritizing the rapid processing of individual targets.
Q2: How do you maintain hormone stability during sample preparation?
A2: Customized extraction workflows adapt to each sample's characteristics. Initial solvent-based separation removes target compounds from plant matrices, followed by SPE purification stages. Quantification accuracy gets reinforced through isotope-labeled reference compounds that account for extraction efficiency variances.
Q3: Do you create custom methods for new hormone analysis?
A3: Absolutely. Our team of engineers tailored detection strategies for emerging phytohormones. Share structural details and experimental objectives - we'll conduct feasibility assessments before developing analyte-specific protocols matching your research parameters.
Customer Review
Detailed Reports and Data Analysis
"The analysis report's depth impressed our team. Visual data representations like chromatogram overlays, paired with statistical breakdowns, transformed complex datasets into actionable agronomic insights. Contextual explanations linking hormone ratios to plant stress responses proved particularly valuable for our field trials."
Customized Sample Preparation Solutions
"The sample preparation protocols were highly optimized for my specific plant tissue. I had previously struggled with hormone degradation, but Creative Biolabs' methods ensured minimal loss and accurate quantification."
Reference
-
Almeida Trapp, Marília, et al. "Validated method for phytohormone quantification in plants." Frontiers in Plant Science 5 (2014): 417. Distributed under Open Access license CC BY 4.0, without modification.
Related Services
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on


 Fig.1 Selected reaction monitoring (SRM) chromatograms of phytohormones in samples.1
Fig.1 Selected reaction monitoring (SRM) chromatograms of phytohormones in samples.1

