Creative Biolabs leads the field in innovative cancer therapies, utilizing our pioneering hypoxia-responsive liposome technology. Our mission is to enhance the efficacy of cancer therapies by developing smart drug delivery systems that target the unique challenges presented by hypoxic tumor environments.
Hypoxia is a hallmark of solid malignant tumors, arising from the rapid proliferation of tumor cells, which leads to an acute demand for nutrients and oxygen within the tumor mass. The resulting demand impairs vascular development in the tumor region, creating an irregular microvascular network that triggers inadequate blood perfusion and induces hypoxia within the tumor. Concurrently, hypoxia accelerates the formation of tumor barriers and increases reactive oxygen species (ROS) and cytokines. Hypoxia-responsive nanomedicine delivery systems leverage the enhanced permeability and retention (EPR) effect and structural transformations for controlled drug release in hypoxic areas, presenting an effective strategy for tumor-targeted drug delivery. A deeper understanding of the hypoxic tumor microenvironment's characteristics is instrumental in designing more effective hypoxia-responsive drug delivery systems, enabling more precise tumor diagnosis and treatment.
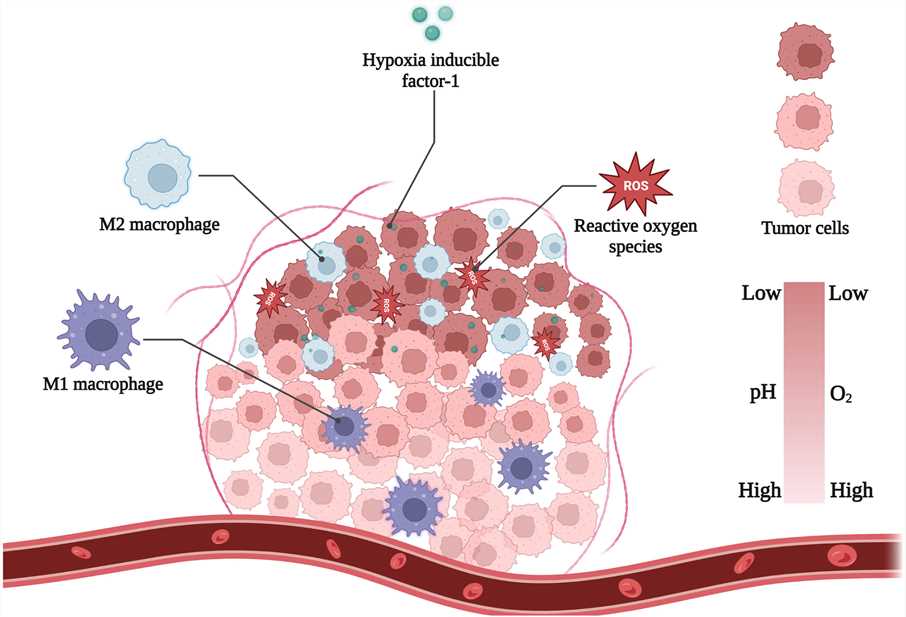 Fig.1 Main hallmarks of hypoxia-tumor microenvironment.1
Fig.1 Main hallmarks of hypoxia-tumor microenvironment.1
Hypoxia-responsive liposome is a type of drug delivery system designed to target hypoxic conditions within the tumor microenvironment. By responding to these conditions, it releases its drug payload, increasing the drug's concentration at the tumor site and enhancing treatment efficacy. Creative Biolabs has developed liposomes that release drugs in hypoxic regions by conjugating hypoxia-responsive chemical bonds to the liposomal structure.
The diversity of hypoxia-responsive chemical bonds under hypoxic conditions endows nanomaterials with distinct functionalities, thereby enhancing their therapeutic and diagnostic efficacy.
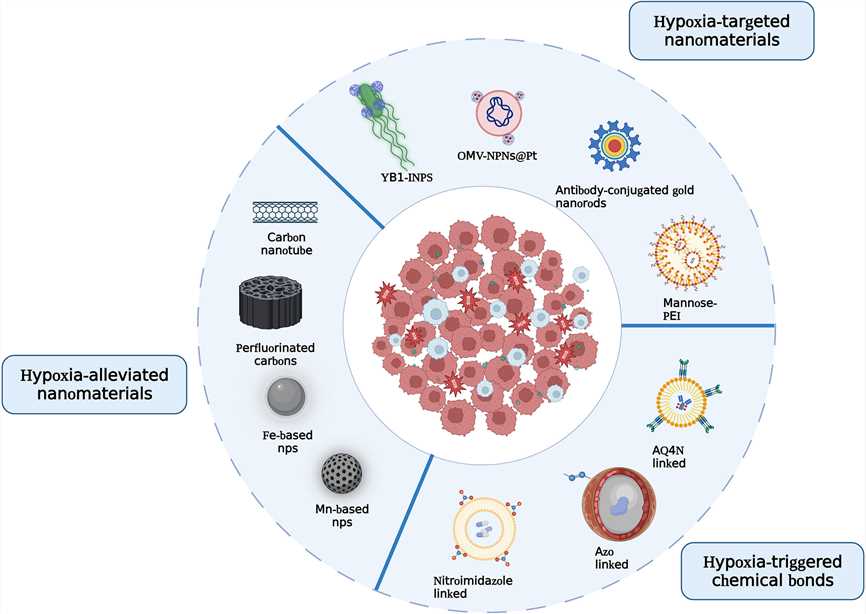 Fig.2 Illustration of the principles to design hypoxia-responsive nanomaterials.1
Fig.2 Illustration of the principles to design hypoxia-responsive nanomaterials.1
Nitroimidazoles are known for their high reactivity under hypoxic conditions and are frequently utilized as hypoxia-responsive components. Within hypoxic tumor cells, nitroreductase and NADPH catalyze a series of bioreductive reactions that sequentially reduce the nitro group (-NO2) to nitroso (-NO), hydroxylamino (-NHOH), and amino (-NH2) groups.
AQ4N undergoes activation and reduction by hypoxic tumor cells to form AQ4, a cytotoxic metabolite. Elevating hypoxic conditions within the tumor microenvironment facilitates the conversion of AQ4N into the noxious AQ4. Glucose oxidase (GOX), an enzyme that depletes glucose and oxygen while generating hydrogen peroxide (H2O2), intensifies hypoxia and oxidative stress within tumor cells. Co-encapsulation of AQ4N with GOX in liposomes effectively suppresses tumorigenesis and progression.
AZO groups are a commonly utilized moiety in the fabrication of hypoxia-responsive liposome, as they can be reduced into two separate aniline groups by NAD(P)H dehydrogenase [quinone] 1 (NQO1) and azoreductase under hypoxic conditions. This reduction not only enhances cellular uptake and accumulation of liposomes in tumors but also allows non-luminescent azo compounds to decompose into luminescent amino derivatives under hypoxic conditions, which can be leveraged for the development of hypoxia-responsive molecular probes and hypoxia-triggered prodrugs. The integration of AZO groups with liposomes facilitates the achievement of improved diagnostic and therapeutic outcomes.
Don't hesitate to contact us and discover the transformative potential of our hypoxia-responsive liposomes in advancing your research endeavors.
Reference
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseServices
Online Inquiry