Creative Biolabs specializes in providing comprehensive services for lipid-based drug delivery systems. We are at the forefront of the industry, offering a full suite of solutions to meet the intricate research needs of today's scientists. Our expertise in Multivesicular liposome (MVL) development is a testament to our commitment to innovation, delivering a cutting-edge technology designed for unparalleled therapeutic efficacy and controlled release.
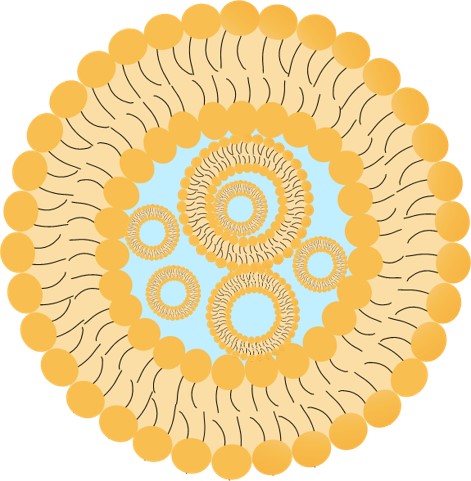
Multivesicular liposomes (MVLs) represent a significant evolution beyond conventional liposomal technology. Defined by their singular, continuous lipid bilayer that encapsulates multiple non-concentric aqueous chambers, this "multivesicular" architecture is a deliberate engineering feat. It provides superior cargo capacity and versatility, enabling the efficient encapsulation and protection of a wide spectrum of therapeutic agents, including challenging molecules like peptides and nucleic acids.
MVLs offer several key advantages and features for advanced drug delivery:
The true genius of MVLs lies in their ability to release their therapeutic payload slowly and steadily. Think of it as a time-release capsule working on a microscopic level. As the drug gently diffuses from its inner chambers, it maintains a stable, effective concentration at the target site. This sustained action not only boosts the therapy's effectiveness but also spares patients from frequent dosing, a significant leap forward for treatments requiring continuous, long-term exposure.
Ready to accelerate your MVL research from lab to life? Creative Biolabs is your innovation engine, providing a powerful, end-to-end service. We handle every critical step, from designing the initial formulation to executing large-scale production, ensuring a solution perfectly tailored to your project. Our expert team of scientists and engineers works alongside you, injecting the specialized expertise needed to navigate the development lifecycle and achieve your goals faster.
The development of MVL is a precise, multi-step process that requires specialized expertise to achieve high stability and EE. We utilize established methods and advanced techniques to ensure the highest quality of every batch.
| Method | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Double Emulsion Method | A w/o/w double emulsion is formed, followed by the removal of the organic solvent. The outer water phase contains a multivalent cation that interacts with the lipids, leading to the multivesicular structure. |
High EE Scalable |
Requires precise control over multiple steps, and residual solvent may be a concern. |
| Cochleate Cylinder Transformation | A continuous lipid bilayer is rolled up into a cylinder ("cochleate") and then transformed into a spherical MVL. | Does not require organic solvents. | The resulting product may contain non-entrapped vesicles that need to be removed via centrifugation. |
| Freeze-Thaw Method | A pre-formed liposome suspension is subjected to multiple cycles of freezing and thawing, inducing membrane fusion and leading to a multivesicular structure. | Simple and does not require organic solvents. | Lower EE and size control can be a challenge. |
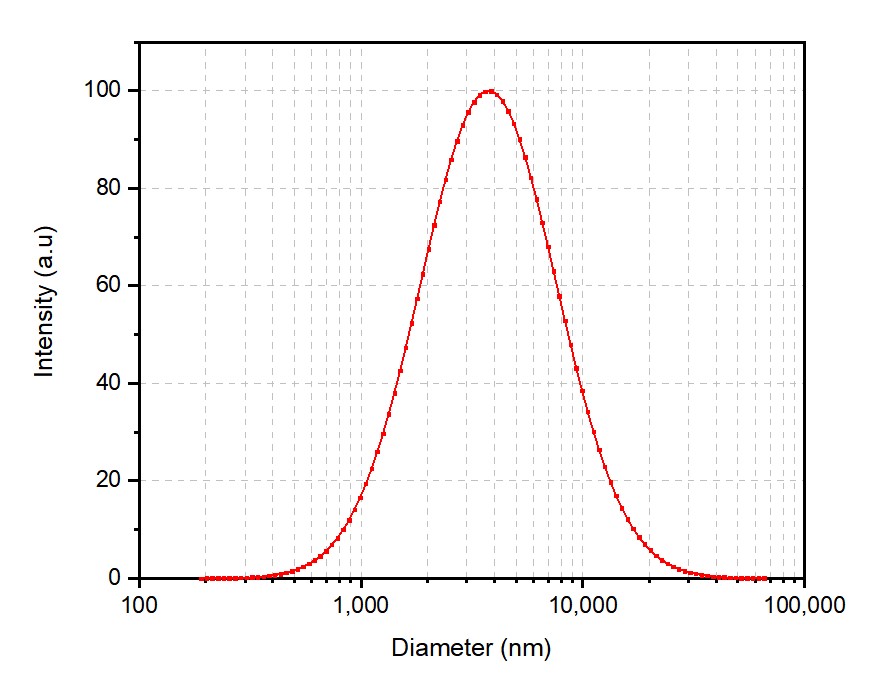
A successful project demonstrates Creative Biolabs' exceptional capabilities in MVL development. We successfully developed an MVL for a client's project that encapsulated an antibody, achieving precise particle size control and high loading efficiency.
Creative Biolabs provides both liquid and freeze-dried (lyophilized) delivery solutions, offering you the flexibility to choose the format that best suits your downstream application and storage requirements.
These results underscore the superiority of our MVL development services. An EE of over 80% is a clear testament to the effectiveness of our formulation and preparation processes, ensuring maximized drug loading and therapeutic potential.
| Step | Description | Starting Materials | Deliverables | Estimated Time |
|---|---|---|---|---|
| 1 | Initial Consultation & Project Scoping | API |
Detailed Project Plan Proposal Quotation |
< 1 Week |
| 2 | Formulation & Process Development | Drug Substances | Optimized liposome formulation | 2-4 Weeks |
| 3 | Production & Purification | Optimized formulation | Comprehensive data on particle size, zeta potential, and EE. | |
| 4 | Comprehensive Characterization | Purified liposome | Detailed COA (Size, Zeta Potential, EE%). | 1-2 Weeks |
| 5 | Delivery | / | Product and COA | < 1 Week |
The unique properties of MVLs make them an invaluable tool across a diverse range of therapeutic and diagnostic fields. Their controlled-release and targeting capabilities can revolutionize treatment approaches, from chronic disease management to personalized medicine.
Our commitment to scientific excellence and client collaboration sets us apart. When you partner with Creative Biolabs, you gain more than just a service—you gain a strategic ally in your research journey.
As a pioneer in the field of lipid-based drug delivery systems, Creative Biolabs has established itself as a trusted partner with decades of rich experience and a profound scientific foundation. Our MVL development service is not just about technology; it's a commitment to your project's success. We are dedicated to providing you with customized, high-quality liposome solutions to accelerate your research and drive innovation in the drug development sector together. We sincerely invite you to contact our expert team to discuss your project needs and begin a collaborative journey towards efficient and successful outcomes.
| Product Name | Description | Inquiry |
|---|---|---|
| Research-Grade Phospholipids | High-purity phospholipids for a wide range of liposome and LNP formulations. | Inquiry |
| Plain Liposomes | Empty liposomes with various standard lipid compositions and proportions, perfect for use as control groups or for comparison. | Inquiry |
| Drug-Loaded Liposomes | Ready-to-use liposomes with a particle size of around 100nm, designed for specific drug encapsulation with customizable particle sizes. | Inquiry |
| Fluorescent Liposomes | Liposomes containing fluorescent dyes for real-time tracking and cellular uptake studies in research applications. | Inquiry |
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseServices
Online Inquiry