Glycoproteins are complex biomolecules essential to various biological processes. These molecules are composed of protein backbones covalently linked to carbohydrate (sugar) chains, a feature that critically influences their structure and function. The glycosylation patterns on glycoproteins play a key role in determining their functionality, affecting processes such as cell-cell communication, immune response, and cellular signaling. Given their importance in biological systems, understanding the structure of glycoproteins is crucial for developing therapeutic interventions, particularly in fields such as drug discovery, vaccine development, and disease treatment. Creative Biolabs offers specialized services, such as Glycoprotein Analysis Services and Glycoprotein Detection Services, to help researchers gain deeper insights into glycoprotein structure and function.
What Is the Structure of Glycoproteins?
Glycoproteins are essential biomolecules formed when carbohydrates are covalently attached to proteins. Their structure consists of a protein core and one or more sugar chains, which are typically added to the protein during post-translational modifications. These sugar chains can be categorized into two main types: N-linked glycoproteins and O-linked glycoproteins. For N-linked glycoproteins, the sugar chain is attached to the nitrogen atom of an asparagine (Asn) residue within the protein, whereas in O-linked glycoproteins, the sugar chain attaches to the oxygen atom of a serine (Ser) or threonine (Thr) residue. The sugar chains in glycoproteins usually contain 3 to 10 monosaccharides, including common sugars like glucose, galactose, mannose, fucose, N-acetylneuraminate, N-acetylgalactosamine, and xylose. These sugars are linked in a variety of ways to form intricate structures that are crucial for the proper functioning of glycoproteins in biological processes.
Spatial Conformation of Glycoproteins
The spatial conformation of glycoproteins is heavily influenced by the glycan chains attached to the protein backbone. These carbohydrates not only extend out from the protein surface but also play a critical role in the folding and overall stability of the glycoprotein. Glycosylation affects both the tertiary (3D shape) and quaternary structure (subunit organization) of glycoproteins by introducing spatial restrictions or enhancing flexibility. The attachment of sugar chains can affect the way a glycoprotein folds during synthesis, with certain sugar modifications promoting proper folding and preventing aggregation. Additionally, the size and shape of the glycan chains can impact the protein-protein interactions and cellular localization of glycoproteins. This makes glycosylation a key factor in maintaining the functional and structural integrity of glycoproteins, especially for those involved in cellular recognition or immune responses.
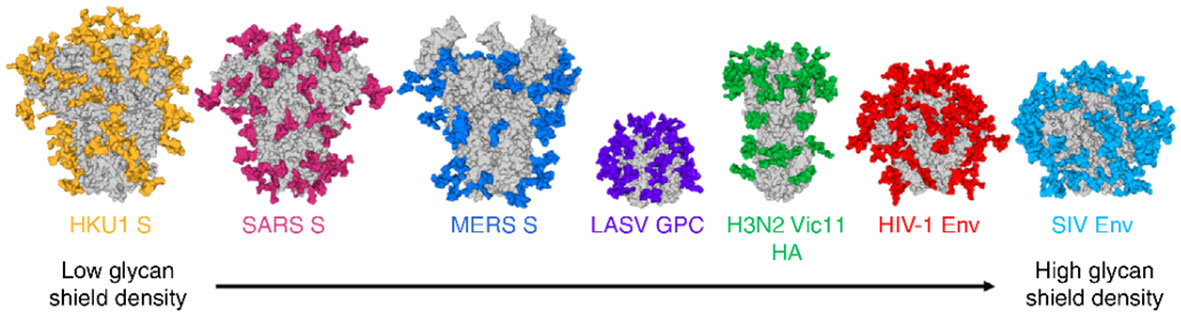 Fig.1 Typical high shielding effects of N-linked glycoepitopes (colored) of common viral envelope glycoproteins.1
Fig.1 Typical high shielding effects of N-linked glycoepitopes (colored) of common viral envelope glycoproteins.1
Distribution of Glycan Chains in Glycoproteins
The distribution of glycan chains in glycoproteins can vary significantly depending on the type of glycoprotein, its function, and the cell or tissue where it is expressed. These glycans are often distributed in distinct patterns along the protein surface and can serve various functional roles based on their size, branching, and composition. For example, glycoproteins involved in cell signaling often have glycan chains that are clustered or more evenly distributed across the protein surface to facilitate interactions with other cell surface receptors or signaling molecules. In contrast, glycoproteins found in cell membranes often have glycan chains extending outward, forming a glycan shield that is involved in cell recognition and immune evasion. The length and branching of the sugar chains can also impact the function of the glycoprotein. For example, highly branched glycans may be involved in protecting the protein from degradation, while simpler, linear glycan chains may be important for interactions with specific ligands or receptors. Moreover, the distribution of these glycans can influence the biological activity of the glycoprotein, such as its role in immune modulation, signal transduction, or protein trafficking.
N-linked vs O-linked Glycoproteins
Glycoprotein structure is deeply influenced by the types of glycosylation modifications they undergo. These modifications not only affect the protein's folding and conformation but also determine its functional diversity. Glycosylation is a fascinating and vital process that happens after a protein is made, where sugar chains (called glycans) are added to the protein. This modification can happen in two main ways: through N-linked glycosylation or O-linked glycosylation, and both have a major impact on how the protein behaves, how it's structured, and what it can do in the body. Think of it like adding a unique tag or decoration to a protein that tells it how to fold, what role it plays in the cell, and even how it interacts with other molecules. Glycosylation is behind many essential biological processes, like making sure proteins fold correctly, stay stable, send signals between cells, and even help the body recognize invaders. In the following sections, we'll take a closer look at how exactly glycosylation shapes these proteins and how the two forms—N-linked and O-linked—contribute to their function.
N-linked Glycosylation Mechanisms
N-linked glycosylation begins in the endoplasmic reticulum (ER), where a sugar chain, called an oligosaccharide, is added to the protein. This sugar chain starts as a fairly complex structure made up of Glc₃Man₉GlcNAc₂ (a combination of glucose, mannose, and N-acetylglucosamine), and it is transferred from a lipid carrier (called dolichol phosphate) to a growing protein chain by an enzyme complex known as oligosaccharyltransferase. For the sugar chain to be added, the protein must have a specific sequence: Asn-X-Ser/Thr, where "Asn" stands for asparagine, and "X" can be any amino acid except proline. Once this sugar chain is transferred to the protein, it undergoes further trimming and modifications in the Golgi apparatus, where additional sugars (like galactose, fucose, or sialic acid) may be added. These modifications are essential for the protein's proper folding, stability, and trafficking within the cell.
O-linked Glycosylation Mechanisms
Unlike N-linked glycosylation, O-linked glycosylation doesn't start in the ER. Instead, it begins in the Golgi apparatus. In this case, the modification starts with the addition of a single sugar called N-acetylgalactosamine (GalNAc) to the hydroxyl group of a serine (Ser) or threonine (Thr) residue in the protein's backbone. This initial sugar addition is carried out by GalNAc transferases. Once the GalNAc residue is in place, the glycan structure can be further extended with additional sugars such as galactose, fucose, and sialic acid, depending on the protein's needs. O-linked glycans tend to be shorter and more varied than their N-linked counterparts, making them highly diverse in structure. However, unlike N-linked glycosylation, O-linked glycosylation does not require a fixed sequence to attach the sugar. Instead, it is a more flexible process, with sugars being added wherever serine or threonine residues are available.
Differences between N-linked vs O-linked Glycosylation
Understanding the differences between these two types of glycosylation provides insight into how glycoproteins function in various biological processes, from immune responses to cell signaling. To further explore these distinctions, let's go through the key differences and functions of N-linked and O-linked glycosylation.
|
Feature
|
N-linked Glycosylation
|
O-linked Glycosylation
|
|
Attachment Site
|
Asparagine (N) residue
|
Serine (S) or threonine (T) residue
|
|
Linkage Type
|
Glycan is linked to the nitrogen atom of asparagine.
|
Glycan is linked to the oxygen atom of serine or threonine.
|
|
Glycan Chain Structure
|
Typically large, branched oligosaccharides (e.g., N-acetylglucosamine, mannose).
|
Usually short, simpler sugar chains (e.g., GalNAc).
|
|
Location of Glycosylation
|
Occurs in the endoplasmic reticulum (ER) and Golgi apparatus.
|
Occurs primarily in the Golgi apparatus.
|
|
Enzymes Involved
|
oligosaccharyltransferase in the ER and various glycosyltransferases in the Golgi.
|
GalNAc transferases and other glycosyltransferases in the Golgi
|
|
Function in Protein Folding
|
Essential for protein folding, quality control, and transport in the ER.
|
Involved in the stabilization of protein structure and modulation of function.
|
|
Functionality in Cell
|
Plays a role in cell signaling, immune response, and protein-protein interactions.
|
Modulates protein function, stability, and subcellular localization.
|
|
Effect on Protein Stability
|
Influences protein stability, prevents aggregation, and assists in trafficking.
|
Modifies protein stability by regulating interactions and protecting from degradation.
|
|
Prevalence
|
More common and involved in the glycosylation of membrane-bound and secreted proteins.
|
Less common, typically found on mucins and cell surface glycoproteins.
|
How to Analyze Glycoprotein Structure?
Understanding the structure of glycoproteins requires advanced techniques to examine both the protein and glycan components. Several methods are available to study the intricate structure-function relationship of glycoproteins.
Mass Spectrometry (MS)
Mass spectrometry is one of the most powerful techniques for identifying glycosylation sites and analyzing glycan structures in glycoproteins. The advantages of mass spectrometry include its sensitivity and ability to detect subtle changes in glycosylation patterns, such as glycoprotein modifications that affect protein function. However, mass spectrometry has its limitations, particularly when analyzing highly complex glycoproteins with multiple glycosylation sites. To overcome these challenges, Creative Biolabs offers services like Glycoprotein Quantification Service and Glycan Profiling Service.
X-ray Crystallography and NMR
X-ray crystallography and NMR are techniques that provide a detailed view of the glycoprotein 3D structure. X-ray crystallography is particularly useful for studying glycoproteins in their crystalline form, while NMR allows for the study of proteins in solution, providing dynamic insights into their conformation. These techniques can help map glycosylation sites and understand how glycoprotein folding and conformation affect their biological activity.
Affinity Chromatography and Gel Filtration
Affinity chromatography and gel filtration are techniques used for isolating and purifying glycoproteins, as well as studying their interactions with other molecules. These methods are essential for understanding the glycoprotein-protein interactions and the role of specific glycoprotein domains in these processes.
Glycoprotein Modeling and Computational Methods
Computational tools have made it possible to predict the structural variability of glycoproteins, allowing for the simulation of glycoprotein folding and glycosylation patterns. These methods complement experimental studies, helping to refine our understanding of how glycosylation affects glycoprotein structure and function.
Find more techniques for glycoprotein structure analysis
For detailed glycoprotein structure analysis, Creative Biolabs provides a variety of specialized services, including Glycoprotein Analysis Services and Glycosylation Site Mapping Service, to assist in understanding the complex relationship between glycoprotein structure and function.
FAQs
Q1: What is the relationship between glycoprotein structure and function?
The structure of a glycoprotein is intimately connected to its function. The glycosylation patterns and conformation of glycoproteins influence how they interact with other molecules, affecting biological processes like immune response, signal transduction, and cell recognition.
Q2: How is mass spectrometry used to identify glycosylation sites in glycoproteins?
Mass spectrometry allows researchers to detect specific glycosylation sites by measuring the mass of glycoproteins and identifying the sugar structures attached to them. This method is highly effective for mapping glycosylation patterns and understanding their impact on glycoprotein function.
Q3: What role do glycoproteins play in the immune system?
Glycoproteins in the immune system, such as antibodies, play a critical role in recognizing pathogens and activating immune responses. Their glycosylation patterns influence how they interact with receptors and other immune cells.
Q4: How are glycoproteins used in vaccine development?
Glycoproteins are often used in vaccine development because their glycosylation patterns can be crucial in triggering immune responses. Many viruses, including those causing COVID-19, rely on glycoproteins for host-cell binding, making them key targets for vaccine design.
Q5: How does glycosylation modify glycoprotein functionality?
Glycosylation affects glycoprotein functionality by altering their structure, stability, and interaction with other molecules. These modifications can influence protein folding, stability, and cellular localization, as well as the protein's interaction with ligands and receptors.
Q6: How do changes in glycoprotein structure relate to cancer development?
Abnormal glycosylation patterns are often found on cancer cell surface glycoproteins, influencing tumor progression and metastasis. These changes can affect cellular adhesion, migration, and immune evasion.
Reference
-
Roy, René. "Cancer cells and viruses share common glycoepitopes: exciting opportunities toward combined treatments." Frontiers in Immunology 15 (2024): 1292588. Distributed under Open Access license CC BY 4.0, without modification.
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Typical high shielding effects of N-linked glycoepitopes (colored) of common viral envelope glycoproteins.1
Fig.1 Typical high shielding effects of N-linked glycoepitopes (colored) of common viral envelope glycoproteins.1