N-Linked Glycoengineering Service in Plant Cell
N-Glycosylation in Plant and Mammalian Cells
There are differences in N-glycosylation between plant and mammalian systems, especially in the structure of complex glycans. Plant cells typically synthesize a limited repertoire of N-glycan structures, including the prevalent GnGnXF structures, characterized by β1,2-linked xylose and α1,3-linked fucose. Paucimannosidic MMXF structures synthesized by β-N-acetylhexosaminidases (HEXO) are also common in plants. In certain cases, plant proteins may exhibit Lea structures, characterized by terminal β1,3-galactosylation and α1,4-fucosylation. In contrast, mammalian N-glycans do not contain xylose residues, and α1,6-fucose is attached to the proximal GlcNAc. Mammalian N-glycan characterized by the attachment of β1,4 galactose is absent in plant N-glycans. These distinctions emphasize the necessity for N-linked glycoengineering in plant-based expression systems. Remarkably, the limited glycosylation capacity of plants has proven advantageous for producing proteins with homogeneous glycans.
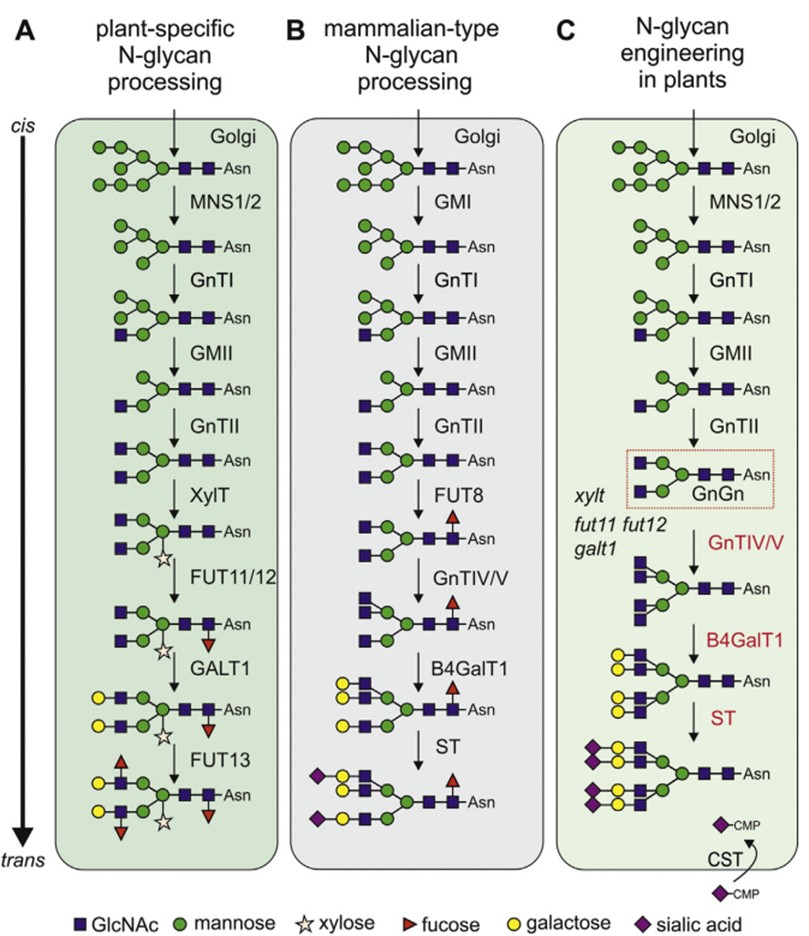 Fig.1 N-glycosylation in plant and mammalian cells.1, 3
Fig.1 N-glycosylation in plant and mammalian cells.1, 3
N-Linked Glycoengineering Services in Plant Cell at Creative Biolabs
By employing cellular engineering techniques such as knockout/knockdown and overexpression of glycosylation enzymes, Plant Cell Glycoengineering Services have been successfully developed and are readily accessible at Creative Biolabs. For N-linked glycoengineering in plant cells, we have devised two main types of modifications. The first is the elimination of plant-specific N-glycans to ensure the homogeneity of recombinant glycoproteins. The second is the introduction of human-like glycosylation pathways, which enables the production of glycoproteins with glycans that mimic the native human glycosylation patterns, resulting in a more biologically relevant product. By combining these strategies, plant expression systems can be customized to yield glycoproteins with desired human-type glycan structures, suitable for various applications.
Elimination of unwanted plant-specific N-glycans
-
Elimination of XylT and FUT11/12 to inhibit the attachment of β1,2-xylose and α1,3-fucose.
-
Elimination of HEXOs to increase complex N-glycans with terminal GlcNAc residues.
-
Elimination of GalT1 and FUT1 to inhibit the synthesis of the Lea epitopes.
Introduction of human N-glycans
-
Introduction of core α1,6-fucose by expression of human α1,6-fucosyltransferase.
-
Introduction of N-glycans containing β1,4 galactose by expressing human β1,4-GalT.
-
Introduction of bisecting GlcNAc by expressing human GnT-III.
-
Introduction of multi-antennary complex glycans by expressing human GnT-IV/V.
-
Introduction of genes responsible for mammalian sialylation pathway.
Advantages of Our Services
-
Highly efficient and functional knockdown and knockout
-
High-level expression vector for overexpression
-
Multiple and comprehensive strategies of glycoengineering
-
Extensive experience in cell line glycoengineering
Published Data
Technology: Plant cell glycoengineering
Journal: Frontiers in Plant Science
IF: 6.627
Published: 2014
Results: The researcher utilized several methods involving the knock-out or knock-down of plant-specific β1,2-XT and core α1,3-FT and introduction of GnTIV and GnTV, β1,4-GalT, and the biosynthetic pathway for sialylation, to transform the glycans present in Nicotiana benthamiana wild-type plants into a glycosylation profile that resembles human serum Erythropoietin.
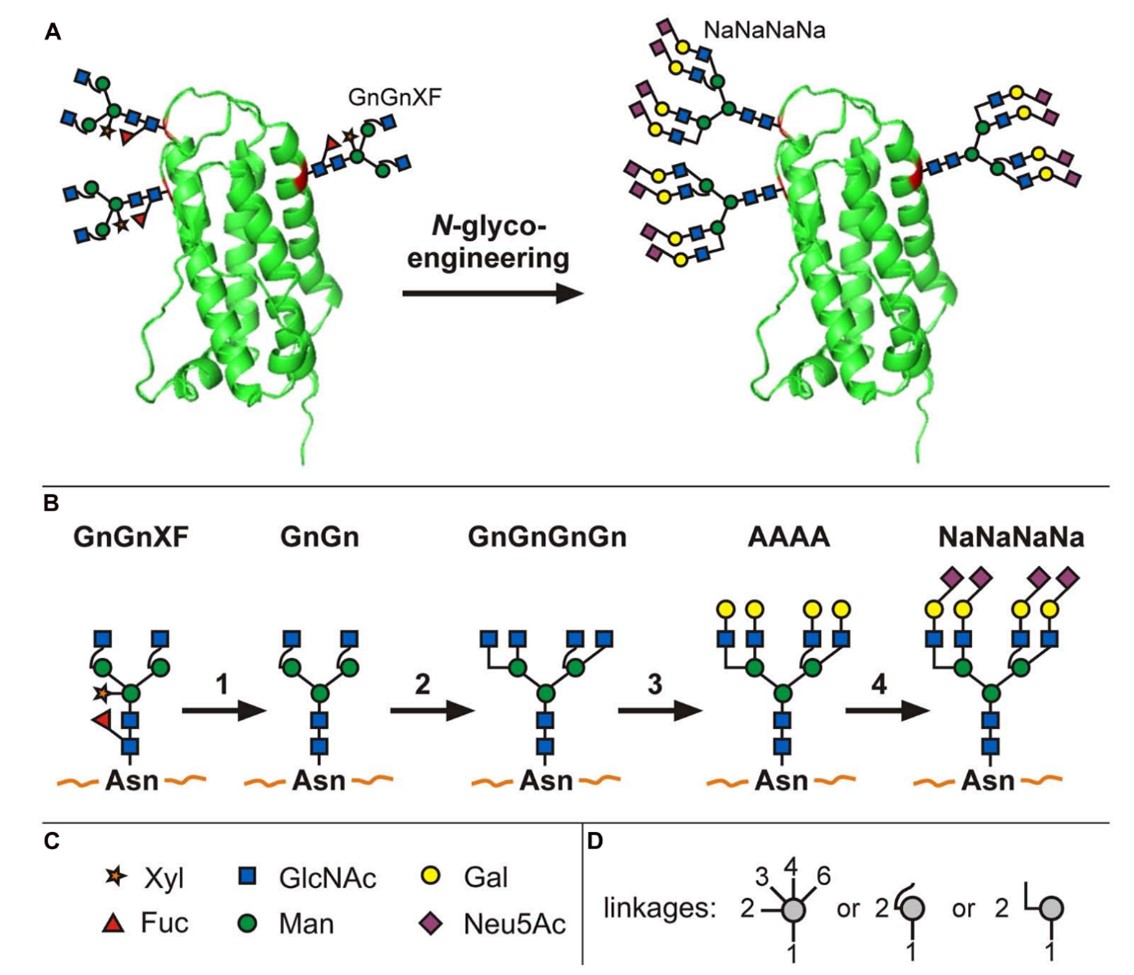 Fig.2 N-glycoengineering in plants to produce tetra-sialylated proteins.2, 3
Fig.2 N-glycoengineering in plants to produce tetra-sialylated proteins.2, 3
Creative Biolabs offers comprehensive plant glycoengineering services, allowing for tailored manipulation of glycosylation processes. For further details or specific requirements, please don't hesitate to contact us.
References
-
Schoberer, Jennifer, and Richard Strasser. "Plant glyco-biotechnology." Seminars in Cell & Developmental Biology. Vol. 80. Academic Press, 2018.
-
Loos, Andreas, and Herta Steinkellner. "Plant glyco-biotechnology on the way to synthetic biology." Frontiers in plant science 5 (2014): 523.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 N-glycosylation in plant and mammalian cells.1, 3
Fig.1 N-glycosylation in plant and mammalian cells.1, 3
 Fig.2 N-glycoengineering in plants to produce tetra-sialylated proteins.2, 3
Fig.2 N-glycoengineering in plants to produce tetra-sialylated proteins.2, 3

