O-Linked Glycoengineering Service in Bacteria
O-Glycosylation in Bacteria
To date, multiple O-glycosylation mechanisms have been identified across diverse bacterial species. These mechanisms are categorized based on their reliance on O-oligosaccharyltransferases (OSTs) into two main types: OST-independent and OST-dependent glycosylation. In the OST-independent pathway, glycosyltransferases (GTs) work sequentially in the cytoplasm to add individual monosaccharides to acceptor proteins. OST-dependent glycosylation involves the assembly of an oligosaccharide on a lipid carrier Und-PP. This oligosaccharide is then transferred en bloc by an OST to specific serine or threonine residues within acceptor proteins. The ability of OSTs to catalyze glycan attachment to natural O-glycoprotein targets in E. coli provides evidence of their potential to transfer non-native glycans to target proteins in glycoengineered bacteria. This capability opens up new possibilities for engineering glycosylation pathways in bacterial systems to produce glycoproteins with tailored glycan structures for various applications.
O-Linked Glycoengineering Services in Bacteria at Creative Biolabs
The identification of OSTs with relaxed specificity has significantly broadened the toolbox available for O-linked glycoengineering in bacteria. With a wealth of expertise in Cell Line Glycoengineering, Creative Biolabs is dedicated to implementing a variety of biosynthetic strategies, with the aim of engineering bacteria to mimic human-like O-glycosylation pathways. Through the co-expression of heterologous GTs and OSTs, we have achieved different human glycoproteins with the site-directed attachment of O-glycans such as the human mucin-type O-glycans.
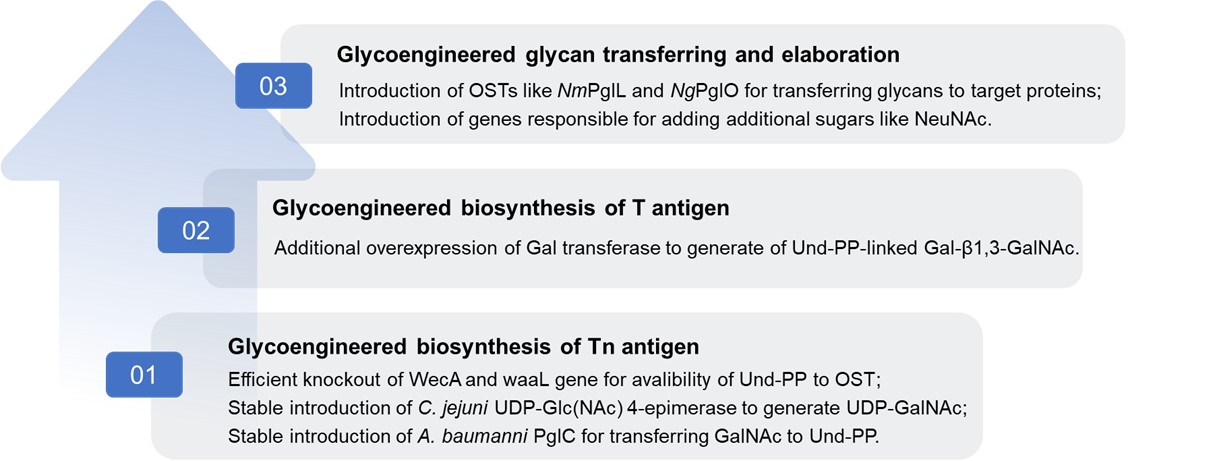 Fig.2 Glycoengineering in bacteria for human mucin-type O-glycans.
Fig.2 Glycoengineering in bacteria for human mucin-type O-glycans.
These modifications by Genetic Glycoengineering Techniques enable the stepwise biosynthesis of specific mucin-type O-glycans. We provide robust platforms in O-linked glycoengineering in bacteria for producing glycoproteins with defined O-glycosylation patterns, which allow for the customization of O-glycan structures to meet various research and application needs.
Features of Our Services
-
Comprehensive profiling of enzymes involved in bacterial O-linked glycosylation
-
Multiple strategies for O-linked glycoengineering in bacteria
-
Advanced techniques for highly efficient knockout/knockin of target gene
-
Customized optimization for specific O-glycans
-
Professional team with extensive experience in glycoengineering
Published data
This study aims to develop a novel synthetic biology strategy for the production of bioconjugate vaccines to replace the traditional N-glycosylation method. The authors reported an O-linked glycosylation system that utilized Neisseria meningitidis, which could efficiently transfer a variety of glycans to proteins to produce effective conjugate vaccines. Studies have shown that this O-linked glycosylation system could not only glycosylate different protein substrates but could also be effectively used in other Gram-negative bacteria. Animal experimental results showed that the conjugate vaccine produced by this system induced potential protective antibody responses. This research result provided a simpler and more powerful strategy for the production of bioconjugate vaccines against various pathogens and expanded the application prospects of O-linked glycosylation.
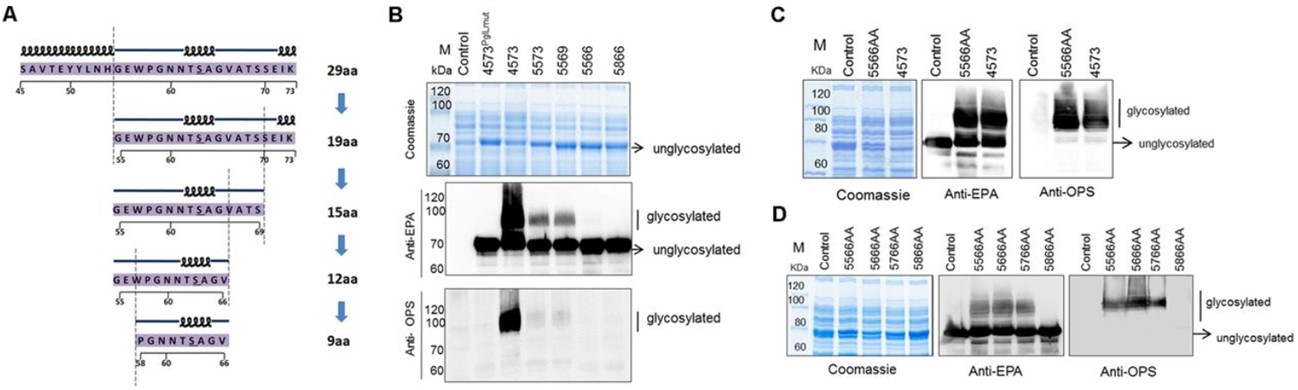 Fig.1 O-linked glycosylation system-based determination of the minimum recognition motif.1
Fig.1 O-linked glycosylation system-based determination of the minimum recognition motif.1
Creative Biolabs offers a comprehensive range of O-linked glycoengineering services in bacteria customized to meet a variety of application requirements. If you have any specific questions or requirements, please feel free to contact us. We look forward to assisting you with your glycoengineering needs.
Reference
-
Pan, Chao, et al. "Biosynthesis of conjugate vaccines using an O-linked glycosylation system." MBio 7.2 (2016): 10-1128. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.2 Glycoengineering in bacteria for human mucin-type O-glycans.
Fig.2 Glycoengineering in bacteria for human mucin-type O-glycans.
 Fig.1 O-linked glycosylation system-based determination of the minimum recognition motif.1
Fig.1 O-linked glycosylation system-based determination of the minimum recognition motif.1

