Lipid-Based Drug Delivery Systems in Influenza Treatment
Background Challenges Creative Biolabs' Solutions Applications Workflow Published Data Related Services Resources
Are you currently facing long drug development cycles, challenges in anti-influenza efficacy, or issues with vaccine delivery in influenza treatment? By improving drug absorption, targeting delivery, and overcoming resistance, Creative Biolabs' lipid-based drug systems are advancing the development of new flu treatments. Our advanced LipoDrive™ platform helps achieve superior therapeutic outcomes and streamline your biopharmaceutical development processes.
Background of Influenza
Caused by specific viruses, influenza (or the flu) is a very contagious condition that mainly affects the respiratory system and comes on suddenly. Influenza viruses are notorious for their significant genetic variability, which allows them to constantly evade host immunity, leading to annual epidemics and unpredictable pandemics. Common signs are a quick start of fever, cough, a sore throat, body aches, headaches, and feeling tired. Complications can range from bacterial pneumonia and acute respiratory distress syndrome (ARDS) to exacerbation of chronic medical conditions, especially in high-risk populations like the elderly, young children, pregnant individuals, and those with compromised immune systems.
Influenza viruses belong to the Orthomyxoviridae family, mainly categorized into A, B, C, and D types according to the antigenic characteristics of their nucleoprotein and matrix protein. For influenza A virus (IAV), subtypes are distinguished by surface components, hemagglutinin (H) and neuraminidase (N), which are essential for viral entry and release. This classification system reflects their genetic variability and distinct epidemiological patterns.
|
Influenza Viruses
|
Characteristics
|
|
Influenza A Virus (IAV)
|
It spreads easily and can lead to illnesses ranging from moderate to severe.Occurs in all age groups
Affects humans and other animals, like birds and pigs
|
|
Influenza B Virus (IBV)
|
Causes milder disease than type A
Affects primarily children
Its main host is humans, and it's classified into two primary lineages known as Victoria and Yamagata.
Seasonal outbreaks
|
|
Influenza C Virus (ICV)
|
Reported rarely in humans
No epidemics
Causes mild respiratory illness
|
|
Influenza D Virus (IDV)
|
Affects cattle and pigs
|
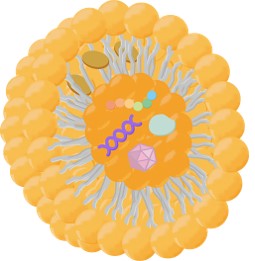
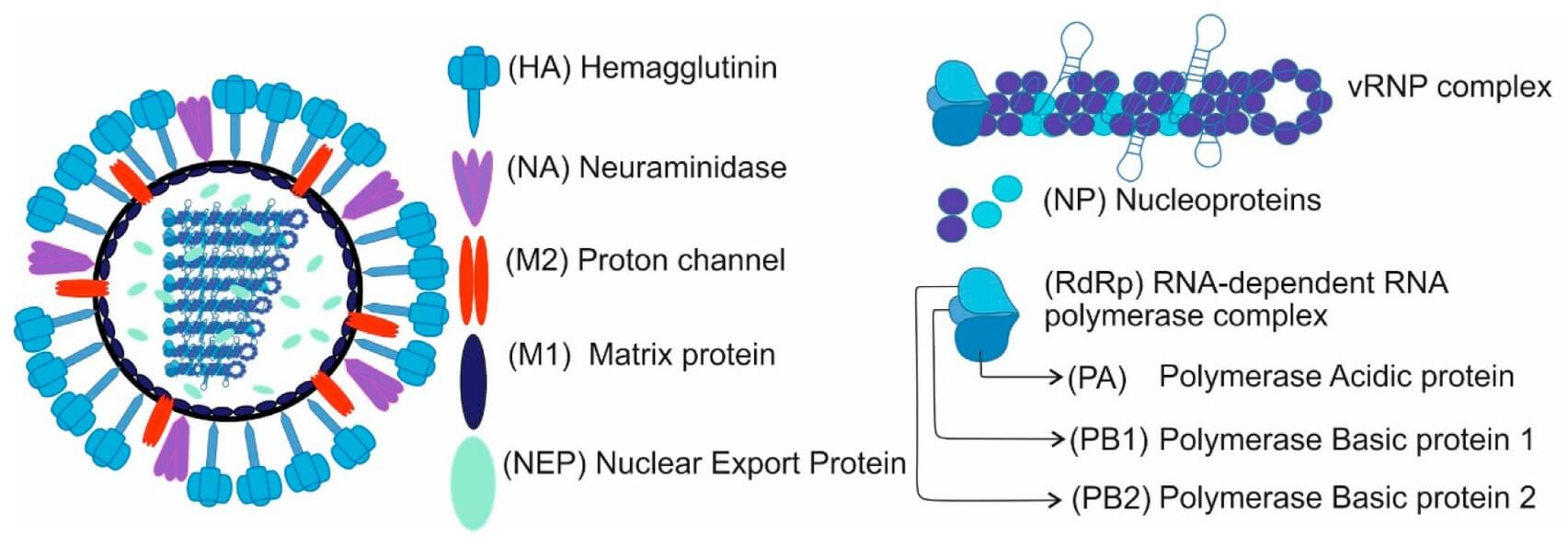 Fig. 1 Scheme of IAV structure.1,3
Fig. 1 Scheme of IAV structure.1,3
Challenges in Influenza Treatment
Current influenza treatment strategies primarily focus on vaccination for prevention and anti-influenza drugs for therapeutic intervention, alongside symptomatic relief. Despite existing strategies, significant challenges persist in influenza treatment:
-
Antiviral Resistance: The rapid evolution of influenza viruses can lead to the emergence of drug-resistant strains, limiting the effectiveness of current antiviral therapies.
-
Narrow Therapeutic Window: Antivirals are most effective when administered very early in the infection, often before diagnosis is confirmed.
-
Systemic Side Effects: Some antiviral drugs can have undesirable side effects, impacting patient compliance and suitability for certain populations.
-
Delivery Limitations: Conventional drug delivery methods may face challenges in achieving optimal drug concentrations at the site of infection (e.g., lungs) while minimizing systemic exposure.
-
Vaccine Efficacy Fluctuations: The need for annual vaccine reformulation due to antigenic drift and shift can lead to varying levels of effectiveness each season.
Creative Biolabs addresses these critical challenges by developing innovative lipid-based drug delivery systems. Our advanced formulations offer solutions for enhanced drug encapsulation, targeted delivery to respiratory cells, improved stability, and the potential to bypass resistance mechanisms, paving the way for more effective and safer influenza treatments.
How Creative Biolabs' Lipid-Based Drug Delivery Systems Can Assist Your Project
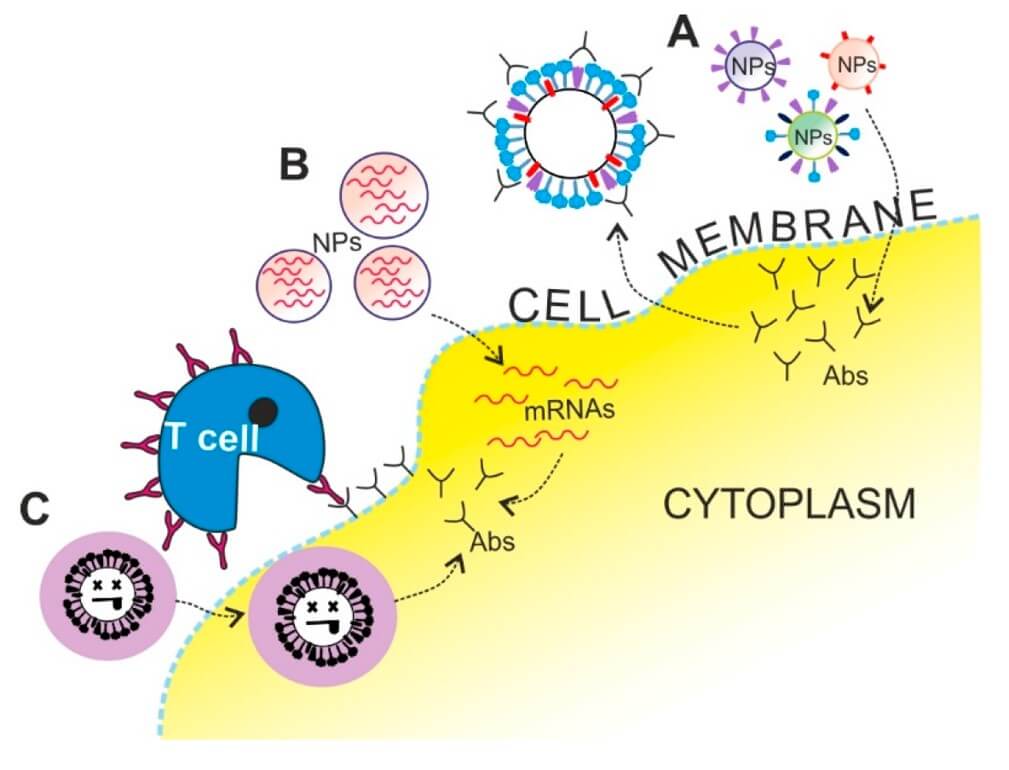 Fig. 2 Schematic representation of different nanotechnology
Fig. 2 Schematic representation of different nanotechnology
applications in vaccination strategies. 1,3
Creative Biolabs' lipid-based drug delivery systems provide transformative solutions for overcoming the inherent challenges in influenza therapeutics and vaccine development. Our expertise in designing and optimizing liposomes and lipid nanoparticles (LNPs) enables enhanced project outcomes through the precise execution of various influenza virus suppression strategies:
-
Advanced Vaccination Strategies: Our lipid-based drug delivery systems are integral to developing next-generation influenza vaccines.
Viral Antigen Vaccination Strategies
We utilize LNPs to encapsulate and deliver viral antigens, enhancing their stability and immunogenicity. This approach ensures more effective presentation of antigens to the immune system.
Inactivated Influenza Virus Strategies
LNPs can serve as sophisticated carriers for inactivated influenza viruses, improving their stability and allowing for controlled release. This enhances the vaccine's ability to elicit a robust immune response while maintaining the safety profile of inactivated vaccines.
mRNA Vaccines Strategies
Our cutting-edge LNP technology is optimized for the stable and efficient delivery of mRNA encoding influenza viral proteins. By allowing in-vivo antigen production that mimics natural infection, this method strongly stimulates both antibody and cellular immunity, marking a significant improvement in vaccine technology.
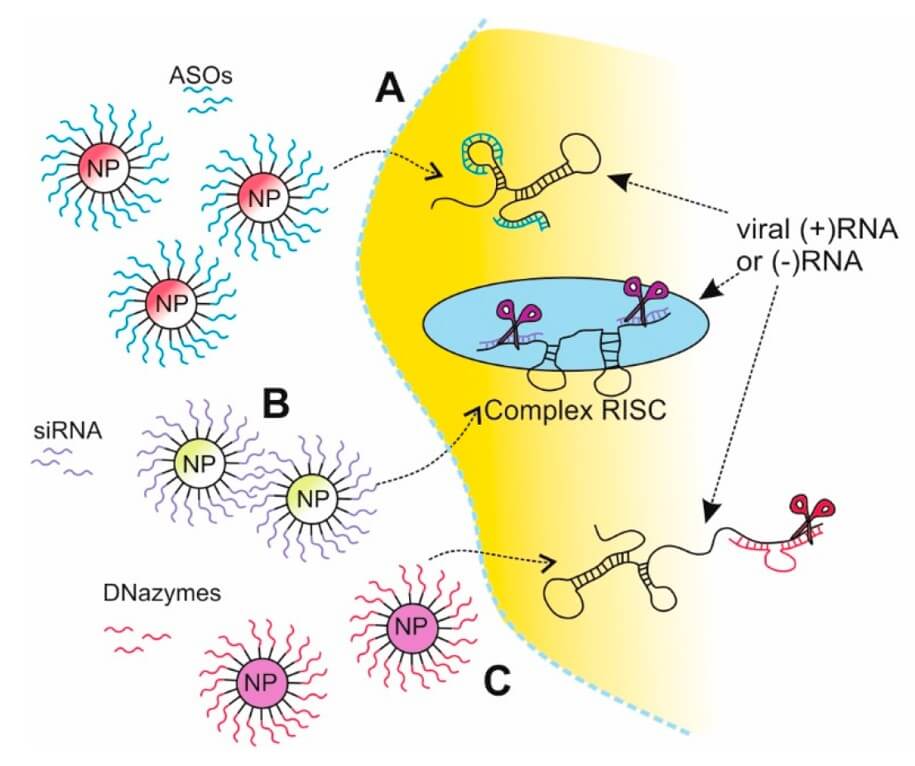 Fig. 3 Schematic view of nanoparticle applications in gene silencing strategies. 1,3
Fig. 3 Schematic view of nanoparticle applications in gene silencing strategies. 1,3
-
Potent Gene Silencing Strategies: Creative Biolabs leverages lipid-based delivery to enable targeted gene silencing, offering novel anti-influenza approaches. The group of silencers includes:
-
Antisense oligonucleotides (ASO)
-
Ribozymes
-
DNAzymes
-
Short interfering RNAs (siRNA)
-
MicroRNAs (miRNAs)
-
Short hairpin RNA (shRNAs)
-
Peptide nucleic acids (PNAs)
ASO Strategy
Our LNPs can effectively deliver ASOs, which are designed to bind to specific viral mRNA sequences, inhibiting viral protein synthesis. This allows for highly specific and targeted suppression of viral replication within infected cells.
siRNA Strategy
We facilitate the delivery of small interfering RNA (siRNA) molecules encapsulated within LNPs. Once delivered to cells, siRNA is incorporated into the RNA-induced silencing complex (RISC), targeting viral mRNAs in a sequence-specific manner. This triggers mRNA degradation, blocks the expression of essential viral proteins, and effectively halts viral spread.
DNAzymes Strategy
Our lipid delivery platforms can be engineered to transport DNAzymes, catalytic nucleic acid molecules that can cleave specific viral RNA targets. This provides another powerful mechanism for disrupting the viral life cycle by directly destroying viral genetic material.
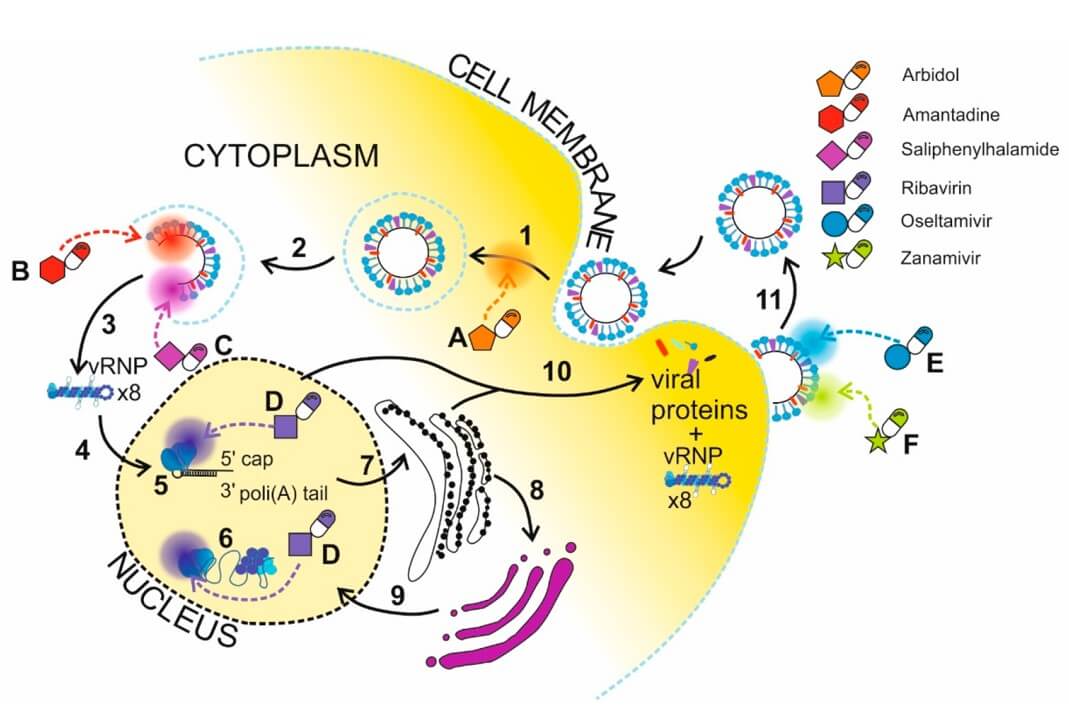 Fig. 4 Schematic representation of anti-influenza drugs
Fig. 4 Schematic representation of anti-influenza drugs
delivery via nanoparticles. 1,3
-
Targeted Drug Delivery for Viral Proteins: Creative Biolabs' lipid-based systems enable precise delivery of small molecule anti-influenza drugs to inhibit critical viral proteins. This ensures that therapeutic agents reach their intended targets within infected cells or specific tissues, maximizing their efficacy and reducing off-target effects.
-
Oseltamivir
-
Zanamivir
-
Ribavirin
-
Amantadine
-
Arbidol
We deliver tailored solutions that translate into more effective therapies and vaccines for influenza. Discover How We Can Help -Request a Consultation.
Potential Applications in Influenza Research
Creative Biolabs' lipid-based drug delivery systems technology offers versatile applications across various stages of influenza research.
In Vitro Studies
Our delivery systems can be readily incorporated into cell culture models to investigate the intracellular trafficking, uptake efficiency, and efficacy of encapsulated antiviral compounds.
In Vivo Studies
In animal models of influenza infection, our delivery systems enable researchers to precisely track drug distribution, assess pharmacokinetics and pharmacodynamics.
Vaccine Adjuvants & Antigen Delivery
Beyond therapeutics, our lipid carriers hold significant potential as adjuvants or antigen delivery vehicles for influenza vaccines.
Workflow for Lipid-Based Drug Delivery Systems Development for Influenza
Published Data
Recent research in Frontiers in Immunology provides compelling evidence for LNP effectiveness against influenza. The study showed LNPs loaded with an RNA adjuvant significantly reduced lung viral replication in mice infected with a matched flu strain, achieving very low viral levels by day 5 post-infection. This protection was linked to robust antibody and T-cell responses. Notably, the specific LNP ionizable lipid composition influenced the type of immune response and lung inflammation, highlighting its critical role in vaccine formulation.
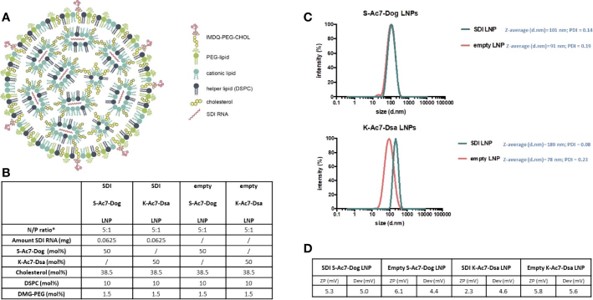 Fig. 5 Structure and characterization of LNPs consisting of K-Ac7-Dsa or S-Ac7-Dog lipids. 2,3
Fig. 5 Structure and characterization of LNPs consisting of K-Ac7-Dsa or S-Ac7-Dog lipids. 2,3
At Creative Biolabs, we offer high-quality LNPs and liposomes, reflecting these successful designs. Our platforms are ideal for researchers studying viral infections, immune responses, and antiviral strategies.
Creative Biolabs stands as a leading partner in advancing influenza treatment through our cutting-edge lipid-based drug delivery systems. Our integrated approach, combining scientific acumen with innovative liposome and LNP technology, offers unparalleled solutions for developing highly effective anti-influenza drugs and vaccines. We are dedicated to accelerating your project, ensuring enhanced drug performance, and contributing to the global fight against influenza. If you are interested in our services or would like to discuss a potential collaboration, please don't hesitate to contact us. Together, we can make a difference in the fight against influenza.
Related Services
Resources
References
-
Wieczorek, Klaudia, Barbara Szutkowska, and Elzbieta Kierzek. "Anti-influenza strategies based on nanoparticle applications." Pathogens 9.12 (2020): 1020. doi:10.3390/pathogens9121020.
-
Jangra, Sonia, et al. "Lipid nanoparticle composition for adjuvant formulation modulates disease after influenza virus infection in quadrivalent influenza vaccine vaccinated mice." Frontiers in Immunology 15 (2024): 1370564. doi:10.3389/fimmu.2024.1370564.
-
Distributed under Open Access license CC BY 4.0, without modification.

For Research Use Only. Not For Clinical Use

 Fig. 1 Scheme of IAV structure.1,3
Fig. 1 Scheme of IAV structure.1,3
 Fig. 2 Schematic representation of different nanotechnology
Fig. 2 Schematic representation of different nanotechnology Fig. 3 Schematic view of nanoparticle applications in gene silencing strategies. 1,3
Fig. 3 Schematic view of nanoparticle applications in gene silencing strategies. 1,3
 Fig. 4 Schematic representation of anti-influenza drugs
Fig. 4 Schematic representation of anti-influenza drugs  Fig. 5 Structure and characterization of LNPs consisting of K-Ac7-Dsa or S-Ac7-Dog lipids. 2,3
Fig. 5 Structure and characterization of LNPs consisting of K-Ac7-Dsa or S-Ac7-Dog lipids. 2,3



 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical Use