The therapeutic potential of an active pharmaceutical ingredient (API) encapsulated within liposomes hinges on its successful release from the liposomal carrier, a prerequisite for its subsequent therapeutic action in vivo. Therefore, it is crucial for liposomes to maintain stability until they reach the target site, where the API is then released. Creative Biolabs offers provides rigorous and accurate In vitro release testing (IVRT) services, supporting the development and regulation of liposomal products.
In vitro release (IVR) serves as a critical technical indicator for assessing the quality of pharmaceutical formulations and plays a pivotal role in product development, quality research, and product release. It is typically utilized for a variety of purposes, such as evaluating drug availability during preliminary research, assessing manufacturing processes, identifying key factors that may influence bioavailability, and ensuring batch quality control. Importantly, IVR-based predictions of bioavailability can be made using in vitro-in vivo correlation (IVIVC) mathematical models, an approach that is beneficial for reducing costs and saving time in the development of new drugs.
The study of liposome IVR encounters two significant challenges: replicating IVR conditions and accommodating the multifaceted manners in which liposomes can release their encapsulated APIs. This complexity arises because minor variations in the physicochemical properties of liposomes can significantly impact drug release behavior, meaning there is no one-size-fits-all approach to studying IVR across different liposome formulations. Creative Biolabs is equipped with state-of-the-art instrumentation and extensive experience in IVRT, enabling us to provide fully customized IVRT services tailored to your specific liposome formulations and release conditions.
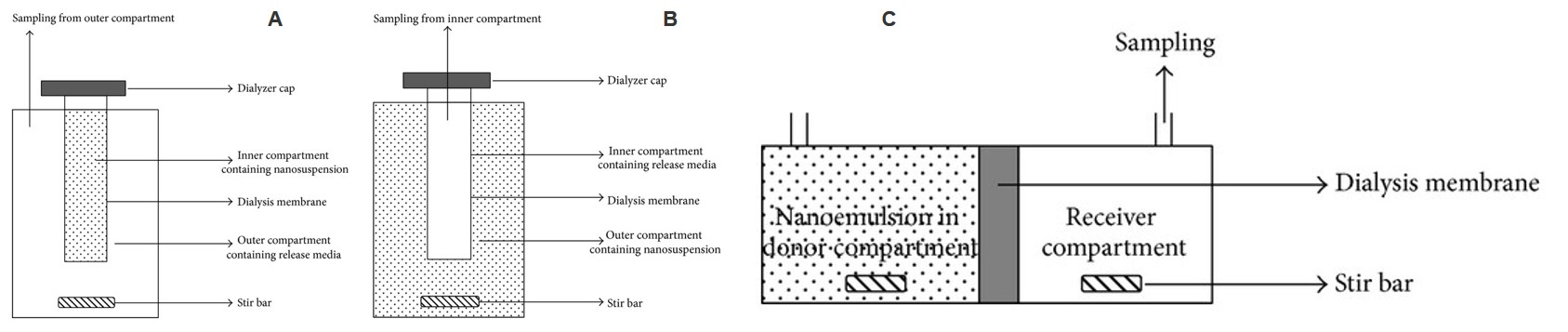 Fig.1 Apparatus for regular dialysis (A), reverse dialysis (B), and side-by-side dialysis (C).1
Fig.1 Apparatus for regular dialysis (A), reverse dialysis (B), and side-by-side dialysis (C).1
| Methods | Details | Advantages | Disadvantages |
|---|---|---|---|
| Sampling Separation | Liposomes are directly placed in the release medium with controlled stirring speed and medium temperature. | Common apparatus, simple sampling, mature method. | Centrifugation or ultrafiltration may disrupt the liposome structure. |
| Dialysis | The liposome suspension is separated from the release medium by a dialysis bag with a specific molecular weight cutoff, allowing the drug to diffuse from the liposomes through the bag into the release medium. | Widely used, low equipment requirements, no need for additional filtering after sampling. | The dialysis bag may adsorb the drug. |
| Ussing chamber | Two perfusion chambers are utilized, each containing the release medium and the test solution, respectively, and are separated by a layer of tissue. Additionally, a piping system is employed for heating and infusing a specific ratio of gases (CO2, O2, or N2). | Maintains membrane integrity and activity during drug release, and simultaneously assesses the drug's permeability and retention within the tissue. | The scope of application is relatively narrow. |
| Franz Diffusion Cell | The apparatus consists of a donor chamber (containing the liposomes) and a receiver chamber (containing the release medium), separated by a membrane. The drug can diffuse through the membrane into the release medium in the receiver chamber. | Low apparatus cost, with no need for additional filtration after sampling. | Bubbles can be challenging to eliminate, which may result in non-uniform drug distribution within the receiver chamber. |
| Flow-through Cell | A constant flow pump is employed to circulate the release medium through a flow-through cell, and the drug concentration in the solution is measured after filtration at the top end. | Prevents medium evaporation, allowing for long-term drug release measurement. | The equipment is costly, the operational procedures are intricate, and the filters are susceptible to clogging. |
| Electrochemical | When liposomes undergo oxidation-reduction reactions at the electrode surface, they generate corresponding current signals. By monitoring the changes in these current signals, the release of the drug can be assessed. | Directly measures drug content, avoiding loss of the drug or liposomes, thereby minimizing errors. | This method is exclusively applicable to APIs that exhibit electrochemical signals. |
Accurate and comprehensive IVR evaluation is essential for optimizing liposome formulations and ensuring their therapeutic efficacy. By developing reliable IVR methods, Creative Biolabs can establish IVIVC models for liposomes, thereby assisting clients in evaluating formulations, assessing manufacturing process parameters, and predicting clinical trial outcomes. If you are interested in our IVRT services or have any questions, please feel free to contact us at any time.
Reference
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseSupports
Online Inquiry