Liposomes, as representative colloidal carriers, have been extensively applied in clinical settings. A suite of analytical and technical methodologies has been developed to characterize their morphology, size, polydispersity index, charge, lamellarity, lipid composition, bilayer fluidity, and encapsulation efficiency. These properties significantly influence the behavior of liposomes both in vitro and in vivo, serving as crucial parameters for their performance evaluation.
Morphology characterization plays a pivotal role in the quality control (QC) of liposomes, as it directly impacts their therapeutic efficacy and performance. Moreover, it is an essential step in ensuring the consistency and reliability of liposomal drug delivery systems. Variations in liposomal morphology can indicate underlying issues such as fusion or aggregation, which are common during liposome storage and can compromise their physical stability.
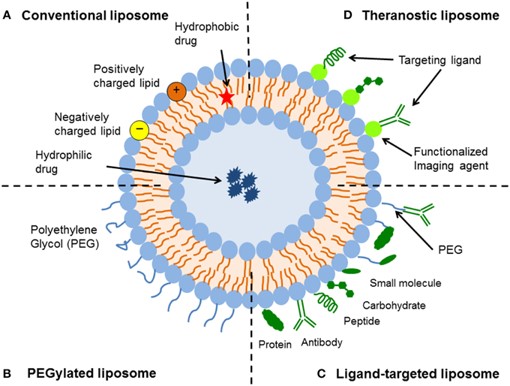 Fig.1 Schematic representation of the different types of liposomal drug delivery systems.1
Fig.1 Schematic representation of the different types of liposomal drug delivery systems.1
The morphological characterization of liposomes is crucial for understanding their behavior both in vitro and in vivo. A diverse array of imaging techniques is at our disposal for the assessment of liposome morphology, each boasting its unique strengths and weaknesses. Choosing an appropriate imaging method depends on the desired morphological features and the resolution necessary for analysis. Gaining insight into these technologies facilitates the selection of an appropriate method for studying nanoparticles based on the specific requirements of a project.
Light or optical microscopy uses visible light and lenses to magnify the field of view, enabling rapid imaging and assessment of liposome characteristics like shape, size, uniformity, and aggregation. Despite its capability to resolve features up to 250 nm, optical microscopy offers insights into the attributes of large vesicles like GUVs, yet it is limited in resolving the intricate structure and layered arrangement of smaller SUVs.
SEM generates magnified images of specimens by scanning them with an electron beam, rather than passing through the sample. This technique can offer general insights into the concentric structures of various lipid layers and details regarding the size and spherical shape of the prepared samples. However, the high vacuum conditions and staining procedures required in the preparation process may disrupt the structure of liposomes.
TEM stands out as the go-to method for scrutinizing nanoparticle structures, achieving high-resolution imaging of delicate samples with resolutions surpassing the 1 nm threshold. The technique can be further classified based on the sample preparation methods used, including negative staining, freeze-fracture, and cryogenic TEM.
Negative staining: It is a relatively swift and straightforward method for preparing samples for TEM. However, when samples are subjected to vacuum conditions, further dehydration can occur, leading to alterations in the vesicle structure. This can result in the appearance of light and dark streaks, which may be mistakenly interpreted as lamellar structures of the liposomes.
Freeze–Fracture: It is a TEM preparation technique that bypasses the need for drying, offering comprehensive 3D structural details of vesicles and their bilayer configurations, particularly highlighting the architecture and packing patterns within multilamellar vesicles.
Cryogenic TEM (cryo-TEM): It is an invaluable tool for analyzing liposomes in their most pristine state, enabling the determination of shape, size, internal structure, and lamellarity. The primary advantage of rapid freezing of liposome samples is the minimization of ice crystal formation, thereby preserving proteins or other materials, which is particularly crucial for the characterization of nanoparticles encapsulating proteins or DNA.
ESEM eliminates the need for fixing, staining, or freezing vesicles, enabling direct imaging of dynamic changes in wet systems without prior sample preparation, thus ascertaining information like vesicle size and shape. Furthermore, ESEM technology enables the observation and analysis of vesicles such as liposomes under various environmental conditions by adjusting the temperature, pressure, and gas composition of the sample environment, which is essential for pharmaceutical formulation and stability research.
AFM, also referred to as scanning force microscopy (SFM), performs in air or liquid, making it a versatile scanning probe technique that forgoes the vacuum requirement. With its superior nanometer-scale resolution, AFM can provide 3D images of liposomes and offer detailed insights into their morphology, size, stability, and uniformity. Additionally, AFM is capable of characterizing ligands on the surface of liposomes, contingent upon the specific conjugation methods employed.
Morphology characterization is an indispensable aspect of ensuring the quality and efficacy of liposomal drug delivery systems. At Creative Biolabs, we offer comprehensive morphology services for liposomes, leveraging SEM, TEM, and cryo-TEM to expertly characterize liposome morphology, ensuring precise and detailed analysis. Contact us to discuss how we can support your specific characterization needs.
Reference
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseSupports
Online Inquiry