Employing effective and reliable research techniques are essential for evaluating liposome stability. At Creative Biolabs, our technical team offers comprehensive liposome stability testing services that are pivotal in the development and optimization of liposome products.
Liposomes serve as sophisticated drug delivery systems, providing advantages such as enhanced drug solubility, targeted delivery, and reduced toxicity. However, their inherent thermodynamic instability leads to challenges like aggregation, fusion, and drug leakage. Addressing these instability issues is paramount to ensuring the efficacy and safety of liposomes during storage and application. Thus, grasping and enhancing the stability of liposomes constitutes a pivotal element in the research and advancement within this domain.
Liposome stability analysis encompasses three pivotal dimensions—physical stability, chemical stability, and biological stability—each examining distinct yet interconnected aspects that collectively determine the liposome's integrity and functionality. Delving into these categories provides a comprehensive framework for understanding the multifaceted nature of liposome stability.
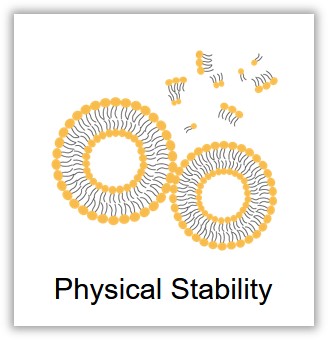
Liposomes are composed of a fluid and dynamic phospholipid bilayer, where phospholipids continuously and freely exchange positions across the membrane. This movement can lead to spontaneous aggregation and sedimentation of the liposome particles, causing instability of the lipid membrane, changes in particle size and zeta potential, and thus affecting the physical structure of the liposomes. Examining the fundamental physicochemical properties of liposomes, such as morphology, particle size, surface charge, and phase transition temperature, is a common approach to assess their stability.
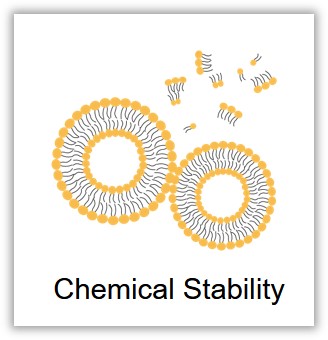
Liposomes are composed of lipids that contain functional groups susceptible to oxidation and hydrolysis. This makes them susceptible to lipid bilayer instability, drug leakage, and the production of toxic byproducts during storage, thereby compromising the stability of the liposomes. Additionally, the chemical stability of liposomes is influenced by factors such as the formulation of the liposomes and storage conditions, including pH, temperature, oxygen levels, and light exposure. To develop more stable liposomes, you can opt for saturated lipids over unsaturated ones and store them in an oxygen-free, low-temperature, and light-protected environment. For further enhancement, Creative Biolabs can incorporate antioxidants during the preparation process or apply a polymer coating to prevent lipid oxidation.
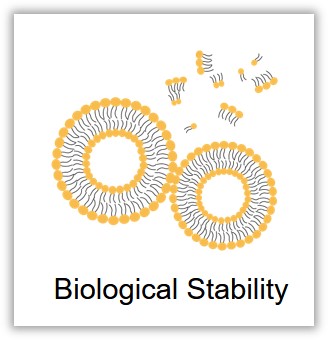
Upon entering the bloodstream, liposomes should maintain their structural and functional integrity until they reach the biological lesion or target site. The complex in vivo environment poses a significant challenge to liposome stability, as various proteins, degradative enzymes, the immune system, and even blood flow can all compromise the stability of liposomes. Conducting in vivo and in vitro assessments of liposome biological stability is not only crucial for the preparation and optimization of liposomes but also provides important evidence for clinical trials and applications.
| Stability Analysis | Detection Indicators | Methods |
|---|---|---|
| Physical Stability | Particle size and size distribution | SEM/TEM, Cryo-TEM, AFM, and dynamic light scattering (DLS) |
| Surface potential and surface pH | Zeta potential measurements with electrical field or pH sensitive probes | |
| Free drug | Gel exclusion chromatography, ion exchange chromatography and protamine precipitation | |
| Drug/phospholipid ratio | Quantitative analysis | |
| Chemical Stability | pH | pH meter |
| Lipid peroxidation | Fatty acid composition (GC) and thiobarbituric acid (TBA) test | |
| Lipid hydrolysis | TLC, HPLC | |
| Antioxidant degradation | TLC, HPLC | |
| Drug degradation | TLC, HPLC, spectroscopy | |
| Biological Stability | Pyrogenicity | Rabbit and/or limulus amebocyte lysate (LAL) tests |
| Animal toxicity | Animal survival rate and cytotoxicity assessment | |
| Drug leakage in various in vivo environments | Gel exclusion chromatography, ion exchange chromatography and protamine precipitation |
A thorough grasp of liposomal systems' physical, chemical, thermodynamic, and biological attributes, along with other pivotal features, enables the rational design of liposomal products. With extensive knowledge in liposome systems, Creative Biolabs offers state-of-the-art stability analysis and formulation strategies like lyophilization and PEGylation, enhancing both the structural integrity and functionality of liposome-based products. Whether for drug delivery, cosmetics, or other applications, our seasoned experts craft tailored stability analysis plans to align with your unique objectives. Contact us promptly to discuss your specific needs and set forth on a trajectory toward a stable and promising future for your liposome formulations.
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseSupports
Online Inquiry