Liposomes are composed of phospholipid bilayers surrounding an aqueous core, with sizes spanning nanometer to micrometer scales. They are widely used as nano-carrier for drug delivery and as cellular model systems for studying membrane properties, transmembrane processes, and intracellular biochemistry. Understanding the lamellarity of liposomes is crucial for optimizing their performance in these applications.
The lamellar structure refers to the number of phospholipid bilayers surrounding the internal aqueous core of the liposome vesicles. This characteristic is a key factor defining both the structure and function of these vesicles and their capacity to accommodate and retain drugs. They are classified by lamellarity into small unilamellar vesicles (SUVs) under 100 nm, large unilamellar vesicles (LUVs) from 100 to 1000 nm, and giant unilamellar vesicles (GUVs) over 1000 nm. Multilamellar vesicles (MLVs) exhibit an onion-like structure made of concentric bilayer membranes.
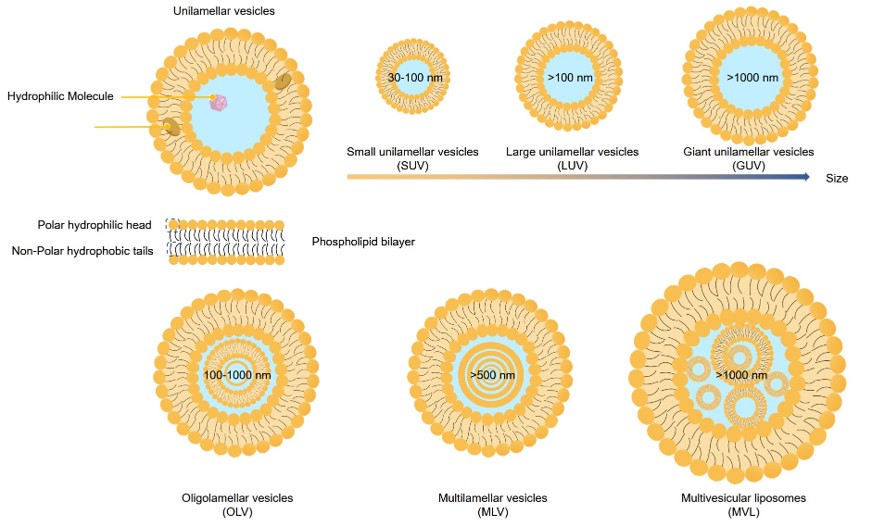 Fig.1 Particle size and lamellarity-based classification of lipid-based drug delivery systems.
Fig.1 Particle size and lamellarity-based classification of lipid-based drug delivery systems.
Lamellarity is a critical factor that influences encapsulation efficiency, liposome stability, release dynamics, and the subsequent behavior of liposomes in cellular interactions. It also governs the mechanical and dielectric properties essential for both cellular engagement and electrokinetic manipulation. In the context of cellular model systems, meticulous regulation of lamellarity is imperative. Typically, unilamellar vesicles (ULVs) exhibit a more rapid drug release profile than MLVs, which provide a greater volume for drug encapsulation. SUVs are often more stable than their larger counterparts due to their reduced surface area, which minimizes the risk of membrane damage. In contrast, MLVs, with their multilayered structure, offer enhanced protection and are generally more stable under adverse conditions.
Table 1. Classification and Characteristics of Liposomes.
| Classification | Size (nm) | Bilayer Count | Characteristics |
|---|---|---|---|
| Small unilamellar vesicles (SUV) | 20-100 | 1 | Small in size, allowing for uniform distribution. They have a small encapsulation volume and low encapsulation efficiency, with a tendency for vesicle fusion. |
| Large unilamellar vesicles (LUV) | 100-1000 | 1 | Possess a large encapsulation volume and high encapsulation efficiency, capable of encapsulating hydrophilic substances. |
| Giant unilamellar vesicles (GUV) | >1000 | 1 | Extremely large in size, offering a vast encapsulation volume and high encapsulation efficiency, suitable for both hydrophilic and lipophilic substances. |
| Multilamellar large vesicles (MLV) | >500 | >5 | Comprise multiple lipid bilayer regions, capable of encapsulating lipophilic substances, but prone to membrane fusion and instability. |
| Multivesicular vesicles (MVV) | >1000 | Multiple | Encapsulate hydrophilic substances and are characterized by low permeability. |
The lamellarity of liposomes is influenced by various factors, including lipid composition and concentration, preparation methods, environmental conditions such as temperature and pH, and microfluidic flow rates. Different lipid compositions and preparation techniques can lead to the formation of distinct types of liposomes, such as SUVs and MLVs. Generally, the use of saturated lipids enhances stability, while unsaturated lipids increase the flexibility of the lipid bilayer but reduce stability.
Liposome lamellarity is typically determined using techniques that monitor the change in visible or fluorescent signals from lipid markers upon the addition of reagents. The assessment is based on the comparison between the overall signal and the signal derived from the interaction between the lipid markers and the specific reagents.
The lamellarity of liposomes is a key determinant of their functionality and performance in various applications. At Creative Biolabs, we are committed to providing the expertise and technology necessary to characterize and optimize liposome lamellarity, ensuring that your lipid-based drug delivery systems and cellular models are as effective as possible. Contact us today to learn more about how our services can support your research and development efforts.
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseSupports
Online Inquiry