Glycan Protein Linkages
Glycoproteins are proteins containing covalently bound oligosaccharides which are composed of saccharide units joined together in a head-to-tail fashion by glycosidic linkages. As a result, each oligosaccharide molecule has one reducing end to be attached to the amino acid residue covalently. Although species-specific glycosylation reactions create a wide diversity of oligosaccharide structures, there are features of N- and O-linked glycans common to all eukaryotes. However, some unusual linkages styles have been found in particular tissue or species. Creative Biolabs put a lot of effort into the glycoprotein area and established a mature platform to provide customized glycoproteins with specific natural glycosylation. Moreover, through the introduction of non-natural amino acids, we developed more novel technologies to add oligosaccharides on proteins without traditional linkages, such as click reaction. We guarantee to provide first-class products and excellent service to customers all over the world.
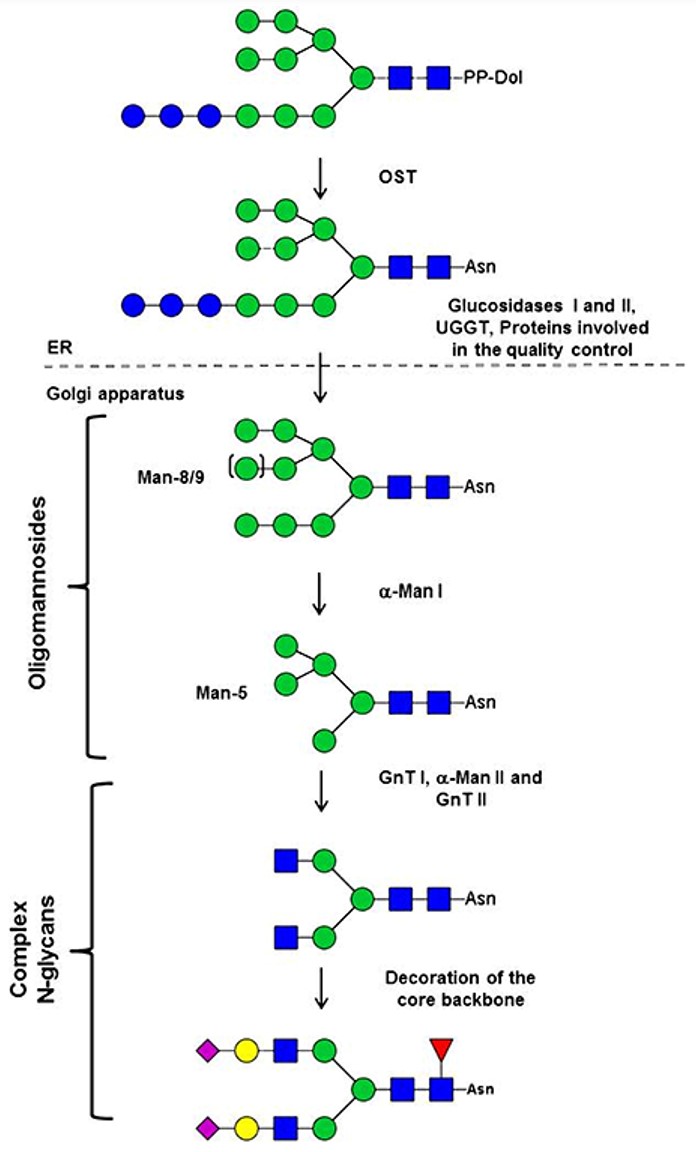 Fig.1 N-glycosylation pathway in mammals.1, 2
Fig.1 N-glycosylation pathway in mammals.1, 2
N-Linked Glycans
The most common protein-linked oligosaccharides are N-glycosidic chains, which are linked to carboxyl on the asparagine residue via N-acetylgalactosamine (GalNAc). N-linked glycans can be further divided into three major classes: the oligomannose type containing N-acetylglucosamine and mannose only; the complex type containing N-acetylglucosamine, mannose, galactose, fucose, and sialic acid; and the hybrid type that has features common to both complex and oligomannose chains.
The pathway for the biosynthesis of N-glycans is initiated by a series of reactions catalyzed by membrane-bound glycosyltransferases found in the endoplasmic reticulum. The beginning Glc3Man9GlcNAc2 glycan was transferred to the nascent peptide chain from a dolichol lipid intermediate. However, subsequent modification of the oligosaccharide chain occurs in the Golgi apparatus. Therefore, despite the diversity, all N-glycans are matured through a similar pathway with a common core glycan structure. The core glycan structure is essentially made up of two N-acetyl glucosamine and three mannose residues. This core glycan is then elaborated and modified further, resulting in a diverse range of N-glycan structures.
O-Linked Glycans
A second glycosylation modification is a covalent attachment of glycans to the α-hydroxyl group of serine or threonine residues to form an O-linked glycan. O-glycans occur on soluble, secreted, and membrane-bound glycoproteins and proteoglycans. Mucins as well as mucin-like glycoproteins are rich in O-glycan chains. Different from N-glycans with conserved core glycan, the only structure common to all O-glycans is the covalent bonding between the hydroxyl group on serine and threonine residues and N-acetylgalactosamine (GalNAc), although xylose, mannose, and other monosaccharides are occasionally found.
O-glycosylation is a post-translational modification that occurs after the protein has been synthesized, which commonly occurs in the Golgi apparatus and occasionally in the endoplasmic reticulum and cytoplasm. Although O-glycans exhibit a high degree of heterogeneity in their structures, eight different core structures are found in various mammalian glycoproteins.
Besides N- and O-linked glycans, many unusual glycosylation types were found in nature such as P-, C- and S- linked glycans. Moreover, many chemical linkage methods have been applied in protein glycosylation due to the development of unusual amino acids introduction. Creative Biolabs provides comprehensive and one-stop glycoprotein research services to our clients all over the world. Please feel free to contact us for more details.
References
-
Mathieu-Rivet, Elodie, et al. "Protein N-glycosylation in eukaryotic microalgae and its impact on the production of nuclear expressed biopharmaceuticals." Frontiers in plant science 5 (2014): 359.
-
Under Open Access license CC BY 3.0, without modification.
For Research Use Only.
Resources

 Fig.1 N-glycosylation pathway in mammals.1, 2
Fig.1 N-glycosylation pathway in mammals.1, 2

