Peptide Moieties
Although oligosaccharide or polysaccharide chains play an important role in the bioactivity of glycoproteins, the main part of glycoproteins is still the peptide chain. Moreover, the structure and folding mode of peptide chains determine the glycosylation accuracy of glycoproteins. Although N- and O-glycosylation always occurs at conserved sites on the protein in mammalian cells, whether it occurs and the monosaccharide unit types of the added glycosylation depends on the structure and distribution of the newly formed peptide and the specificity of glycosyltransferase. Hence, in the expression of the glycoprotein in vitro, it is important to control accurate and stable glycosylation by optimizing the character of peptide moieties of glycoproteins. As a world-leading service provider in the field of glycoproteins, Creative Biolabs can offer comprehensive glycoproteins expression, modification, and analysis services to global clients. We guarantee to provide excellent service and competitive products to customers all over the world.
 Fig.1 Structure of MUC1.1
Fig.1 Structure of MUC1.1
Structural Glycoproteins
Some evidence has indicated that protein-linked glycans not only protect proteins against proteolytic degradation and denaturation but also increase the water solubility. One example of structural glycoproteins is mucins, which are secreted in the mucus of the digestive and respiratory tracts. The oligosaccharide attached to mucins gives them a considerable water-holding capacity and makes them resistant to proteolysis by digestive enzymes. Although some structural glycoproteins occur in connective tissue which helps bind together the fibers, cells, and ground substance of connective tissue. Totally in the structural glycoproteins, the functions are performed by peptide moieties while sugar chains are used to improve the physicochemical properties.
 Fig.2 3D structure of the antibody.2
Fig.2 3D structure of the antibody.2
Immune Glycoproteins
Protein glycosylation has a great impact on the immune system. Almost all key molecules involved in the adaptive and innate immunity such as receptors, immunoglobulins, cytokines, and lectins are glycosylated and some specific glycoforms are involved in recognition events. Most immune glycoproteins are membrane proteins and play important roles in cell recognition. However, the sugar chains of many immune glycoproteins also contribute to the correct fold. For example, there are abundant O-glycosylation sites in the hinge region of an immunoglobulin, which ensure bioactive conformation while enhancing hydrophilicity.
Glycosylated Hormones and Enzymes
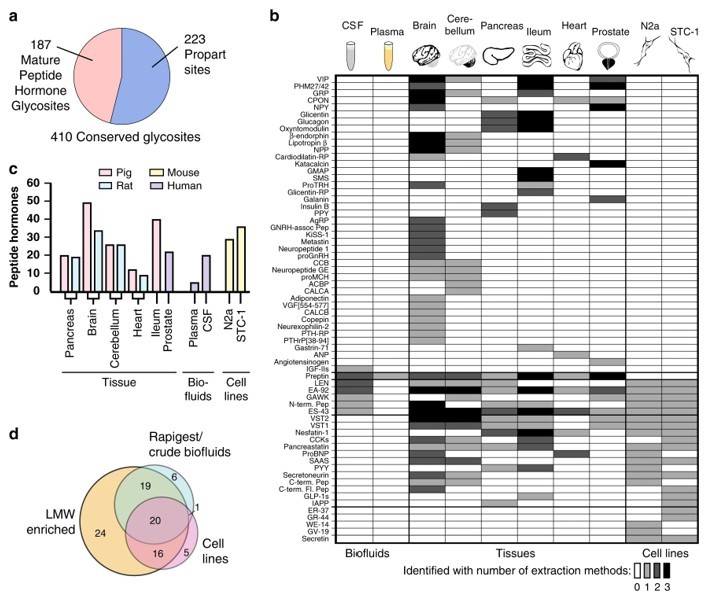 Fig.3 Identification of glycosylated peptide hormones.3, 4
Fig.3 Identification of glycosylated peptide hormones.3, 4
Researches showed that many hormones and enzymes are glycoproteins, in which the sugar chains are important to recognize receptors or substrates, while the subsequent function depends on the peptide moieties. For example, luteinizing hormone and human chorionic gonadotropin are both glycoproteins, which play important roles in human growth. While the phosphorylase is also highly glycosylated to recognize the substrate and attach to a phosphate group.
With the development of protein engineering, it is easy to express the specific amino acid sequence of glycoprotein. However, optimizing peptide moieties to guarantee correct glycosylation is also important to maintain the function of glycoprotein. Creative Biolabs provides convenient and comprehensive glycoprotein-related services to meet every requirement from our global clients. Please feel free to contact us for more details.
References
-
Image retrieved from https://commons.wikimedia.org/wiki/File:Protein_MUC1_PDB_2acm.png, Emw, 2009, used under CC BY-SA 3.0, without any modification.
-
Image retrieved from https://commons.wikimedia.org/wiki/File:Antibody_IgG1_surface.png, Tokenzero, 2020, used under CC BY-SA 4.0, without any modification.
-
Madsen, Thomas D., et al. "An atlas of O-linked glycosylation on peptide hormones reveals diverse biological roles." Nature Communications 11.1 (2020): 4033.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Structure of MUC1.1
Fig.1 Structure of MUC1.1 Fig.2 3D structure of the antibody.2
Fig.2 3D structure of the antibody.2 Fig.3 Identification of glycosylated peptide hormones.3, 4
Fig.3 Identification of glycosylated peptide hormones.3, 4
