Glycoproteins Affect Folding of Certain Proteins
There are relatively few examples of glycoproteins where extensive structural data on both the peptide and glycan components are available. Possibly the most important point to emerge from some examples is the long-range stabilization of the tertiary or quaternary fold of a protein by the addition of a glycan. It is now becoming apparent that N-glycosylation of a folded protein can have a significant stabilizing effect on large regions of the backbone structure. The following are some examples of glycoproteins that affect the folding of certain proteins.
Some Examples of Glycoproteins
Example 1: The Structure of Calnexin was Involved in Quality Control of Protein Folding
Calnexin is a type-I integral membrane protein in the endoplasmic reticulum which coordinates the processing of newly synthesized N-linked glycoproteins with their productive folding. The lumenal domain of calnexin associates with nascent chains of newly synthesized N-linked glycoproteins immediately upon their entry into the lumen of the ER. Several models describing the roles of calnexin and procalreticulin in protein folding have been proposed. The unusual three-dimensional structure with a globular domain and a long extended arm provides a concrete framework for the interpretation of the role of calnexin in protein folding. The two-domain structure of calnexin supports a dual role of calnexin in protein folding. Recent evidence supports a role for calnexin in the direct binding of ERp57, an ER lumenal disulfide isomerase, to both glycosylated and nonglycosylated misfolded proteins. The 3D structure suggests that the novel arm formed by the proline-rich tandem repeats is the most likely site to be involved in interactions with substrate proteins or other proteins of the ER protein folding machinery.
Example 2: Calreticulin’s Function in the Folding of Proteins
Calreticulin is an ER (endoplasmic reticulum) luminal Ca2+-buffering chaperone. Calreticulin and calnexin show a high degree of structural and functional similarities, including domain-like structure. Since the discovery, the protein continues to be implicated in an amazing number of biological systems, including regulation of Ca2+ homeostasis, modulation of transcriptional pathways, cell adhesion, apoptosis, and embryonic development, to name a few. Calreticulin is also involved in the folding of newly synthesized proteins and glycoproteins and, together with calnexin and ERp57, constitutes the “calreticulin/calnexin” cycle that is responsible for folding and quality control of newly synthesized glycoproteins.
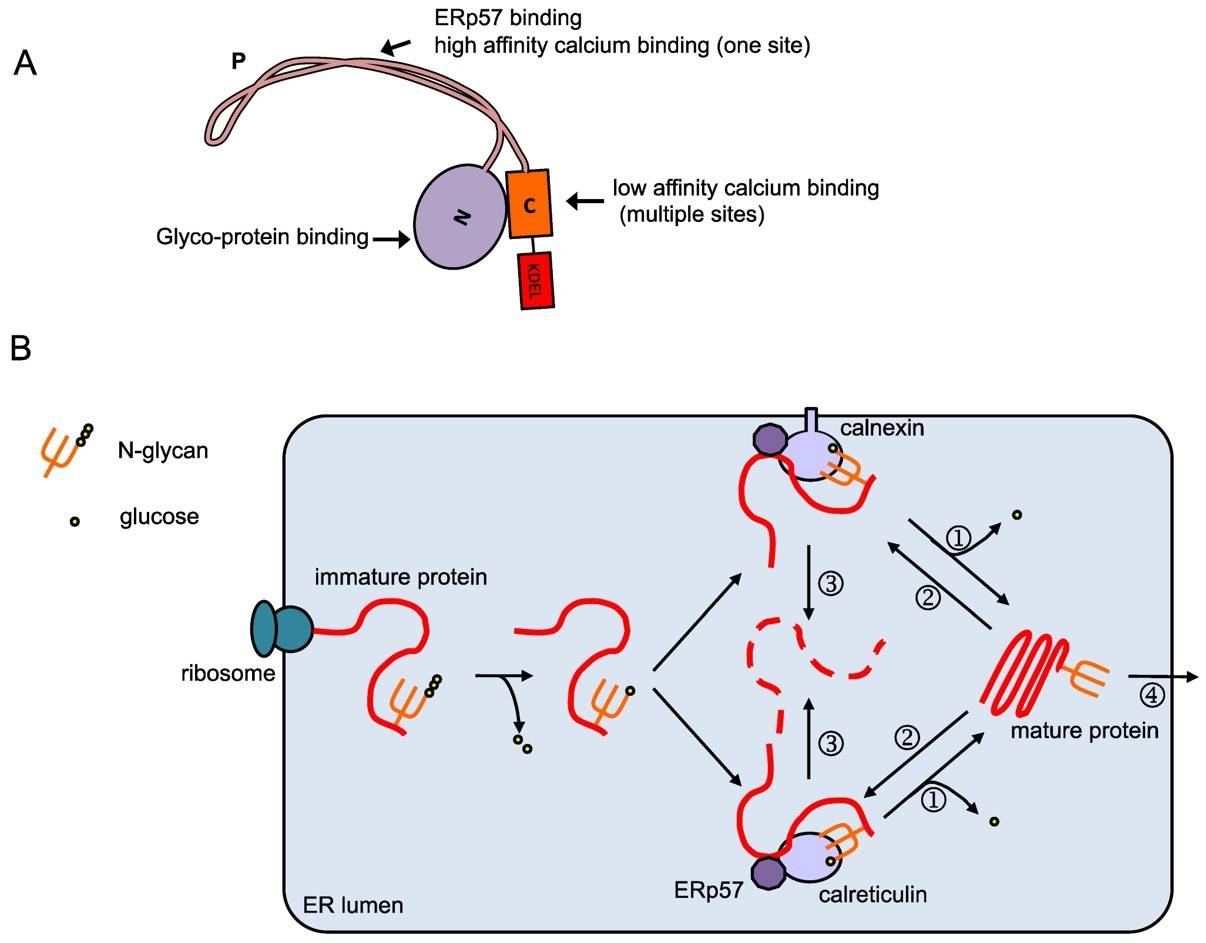 Fig.1 Functions of calreticulin.1, 2
Fig.1 Functions of calreticulin.1, 2
Custom Services for Glycoprotein
Creative Biolabs is a comprehensive and leading biotechnology company. When it comes to glycoprotein research, you need to work with a company with the successful experience to promote the project quickly and effectively. We have accumulated rich project experience, greatly shortened the project cycle, and reduced the cost. If you are interested in our services or technology, please contact us for more details.
References
-
Jiang, Yue, Sandeepa Dey, and Hiroaki Matsunami. "Calreticulin: roles in cell-surface protein expression." Membranes 4.3 (2014): 630-641.
-
Under Open Access license CC BY 3.0, without modification.
For Research Use Only.
Resources

 Fig.1 Functions of calreticulin.1, 2
Fig.1 Functions of calreticulin.1, 2