Glycoprotein Function as Cell Attachment-recognition Site
The fact that most proteins are glycosylated underlies the key roles played by glycosylation during evolution. The functions of glycoproteins are tremendously broad and include cell attachment recognition, homeostasis, transport of molecules, and enzymatic and immunology recognition domains. Here are some examples of glycoprotein with cell attachment recognition function.
some examples of glycoprotein
Example 1: Interaction and Cooperation Between Integrins and Transmembrane Receptors
Integrins are a family of membrane glycoproteins consisting of two subunits, α and β. Both integrin subunits have a large extracellular domain, a transmembrane segment, and a cytoplasmic tail. Integrins belonging to the beta1, alpha v, beta7, and beta4 subfamilies have been shown to potentiate signaling pathways in response to many growth factors, IL-3 and TGFbeta. The cooperation between integrins and other receptors is essential in at least four interlinked processes: (i) receptor transactivation; (ii) receptor coordination; (iii) receptor pathway modulation; and (iv) receptor compartmentalization. Moreover, interference with beta1 integrin/IL-3Rbc interaction, by competitive ligands, led to inhibition of both vasculogenic processes and vessel formation in tumors.
An additional example of reciprocal crosstalk between integrins and transmembrane receptors is represented by morphogens of the TGF-beta family, where TGF-beta upregulates integrin expression and integrins drive TGF-beta activation. Finally, integrins can control the endosomal trafficking of other key receptors such as EGFR and VEGFR2.
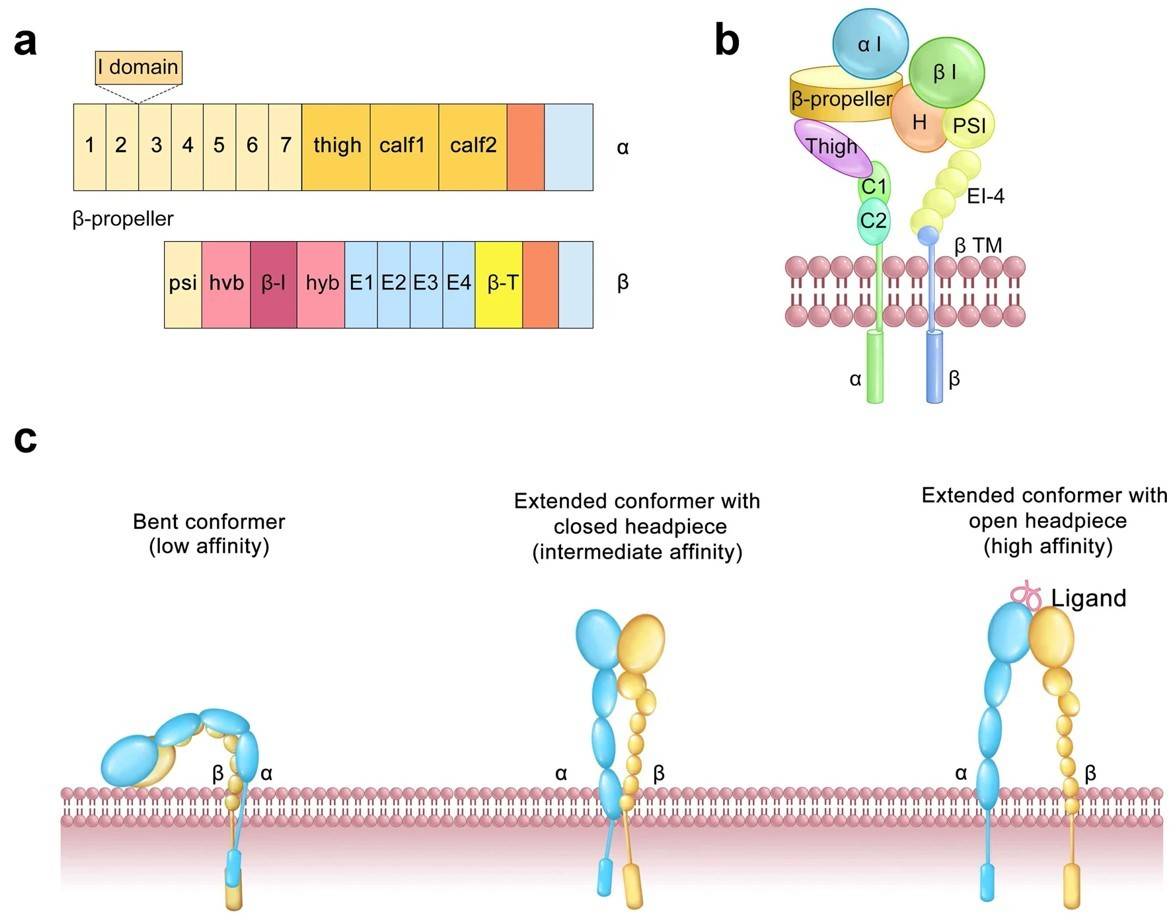 Fig.1 Structure of integrins.1, 2
Fig.1 Structure of integrins.1, 2
Example 2: Ephrin Recognition by NiV-G
The recently emerged Nipah virus (NiV) is an enveloped, negative-sense single-stranded RNA paramyxovirus. NiV contains two membrane-anchored glycoproteins within their envelope, the receptor-binding G glycoprotein (G) and the fusion (F) glycoprotein. The G glycoprotein is a type II membrane protein containing 602-aa residues and, in contrast to most other well-characterized paramyxoviruses, lacks hemagglutinating and neuraminidase activities and does not bind to carbohydrate moieties. The main role of NiV-G is to recognize and attach the virus to receptors within the host cell membrane. The Ephs and the ephrins are divided into two subclasses, A and B, based on their affinities for each other and sequence conservation. All ephrins contain a 20-kDa conserved extracellular Eph-binding domain, which is also recognized by the henipavirus G glycoproteins. The NiV-G/ephrin interactions can be effectively targeted to disrupt viral entry and provide the foundation for structure-based antiviral drug design.
Custom Services for Glycoprotein
Creative Biolabs is a leading biotechnology company specializing in glycoprotein research for many years. When it comes to glycoprotein research, you need to work with a company that has a track record of successful projects and advanced technology. With our experienced experts and flexible project solving ability, we have always been the most wanted partner of scientists. If you are interested in our services or technologies, please contact us for more details.
References
-
Pang, Xiaocong, et al. "Targeting integrin pathways: mechanisms and advances in therapy." Signal Transduction and Targeted Therapy 8.1 (2023): 1.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Structure of integrins.1, 2
Fig.1 Structure of integrins.1, 2

