Glycoprotein Function as Immunologic Molecule
With leading technical strength and a professional team, Creative Biolabs has created a global innovative glycoengineering experimental platform. We are expecting to bring more value to customers with abundant technical resources and customized services.
Glycoprotein Function as Immunologic Molecule
Immune molecules are broadly immunologically active substances. The most representative glycoprotein immune function is immunoglobulin (Ig). It refers to globulins that have antibody activity or chemical structure and are similar to antibody molecules. They are divided into five categories, namely IgG, IgA, IgM, IgD, and IgE. The most important function of Ig is its antibody effect. It can bind antigens (including foreign antigens and self-antigens), thereby effectively removing microorganisms and parasites that invade the body, neutralizing the toxins released by them, or removing some self-aging cells or products. It is an important part of the body's immune system and an important factor in the body’s self-regulation.
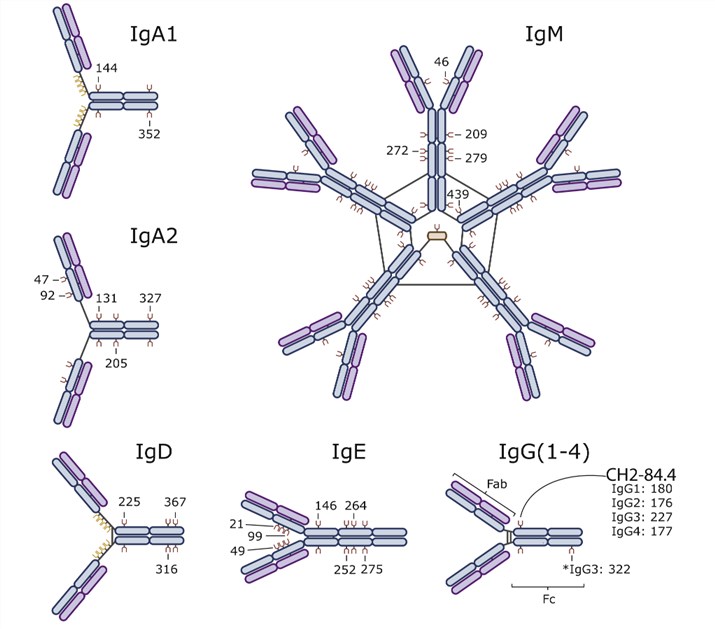 Fig 1. Immunoglobulin classification.1, 3
Fig 1. Immunoglobulin classification.1, 3
A Brief Overview of Antibody Structure
All Igs are comprised of two class-specific heavy chains that are joined together by one or more disulfide bonds. Each heavy chain is also joined by a disulfide bond to a light chain. For IgM and IgA, disulfide bonds can further connect individual Igs to form pentamer and dimer structures, respectively. The antigen-recognition region of Ig is referred to as its Fab fragment. In contrast, the Fc fragment is comprised of the heavy chain region that interacts with the Fc receptors on immune cells. In the IgA, IgD, and IgG isoforms, a flexible linker, which can be decorated with glycans, separates the Fab and Fc regions. IgM and IgE lack this hinge region and are thus more rigid in structure. IgG has a single conserved N-glycosylation site at residue CH2 where large flexible glycans are attached. The other Igs are more heavily glycosylated. These glycan modifications are critical for the appropriate function of all Igs.
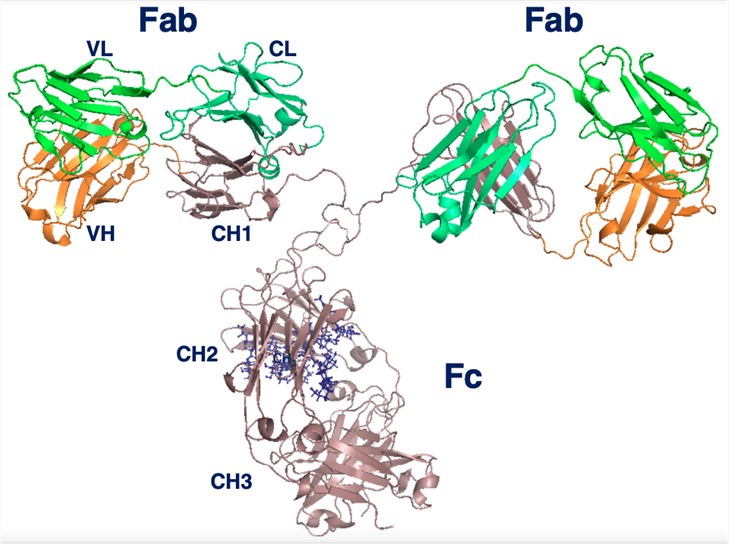 Fig.2 Structural model of a human IgG.2, 3
Fig.2 Structural model of a human IgG.2, 3
The Impact of Glycosylation on Immunoglobulin Effector Function
Since the discovery of altered IgG glycosylation in patients with rheumatoid arthritis, there has been mounting evidence favoring the role of glycans in the pathophysiology of autoimmunity. N-glycans present within the Fc region has been found to help determine the effector function of antibodies. For example, it is now well established that the type of glycan present at residue Asn-180 of IgG1 helps dictate the effector function of the antibody, with some glycans being pro-inflammatory while others possessing anti-inflammatory properties. In addition, studies have shown that glycans are differentially expressed in the setting of autoimmunity and that such differences may be a cause of some autoimmune diseases. Patients with Lambert-Eaton myasthenic syndrome, myasthenia gravis, Crohn's disease, juvenile arthritis, systemic lupus erythematosus, IgA nephropathy and systemic vasculitis have all been shown to have altered Ig glycosylations. This further explains the importance of glycans in the autoimmune system.
Creative Biolabs has a mature team and platform in the field of glycomics, offering a range of Glycoengineering Services such as Therapeutic Glycoprotein Development. If you have questions about glycoprotein immune function, or if you have synthetic glycoprotein requirements, please do not hesitate to contact us for more information.
References
-
Maverakis, Emanual, et al. "Glycans in the immune system and the altered glycan theory of autoimmunity: a critical review." Journal of autoimmunity 57 (2015): 1-13.
-
Chiu, Mark L., and Gary L. Gilliland. "Engineering antibody therapeutics." Current opinion in structural biology 38 (2016): 163-173.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig 1. Immunoglobulin classification.1, 3
Fig 1. Immunoglobulin classification.1, 3
 Fig.2 Structural model of a human IgG.2, 3
Fig.2 Structural model of a human IgG.2, 3

