Glycoprotein Function as Lubricant and Protective Agent
Creative Biolabs is well-known and competitive in the field of glycoengineering. Based on our advanced platform and rich experience, we provide one-stop Glycoengineering Services to customers all over the world.
Introduction of O-linked Glycoproteins
Protein glycosylation is one of the most common post-translational modifications of proteins. And there are two main types of glycosylation of proteins in mammals: N-glycosylation and O-glycosylation. The most abundant form of O-linked glycosylation in higher eukaryotes, termed mucin-type, is characterized by a-N-acetylgalactosamine (GalNAc) attached to the hydroxyl group of Ser/Thr side chains. They can be found in the airway, digestive system, sweat glands, breasts, and even cancer cells. Mucin is a natural barrier that protects the environment surrounding the tissues and cells it is formed by. A mucin polymer is either attached to the cell membrane or part of the gel-like mucus itself. It plays a major role in innate immunity, hydration, lubrication.
The Discovery of Mucin-type O-linked Glycoproteins
The study of mucin-type O-linked glycosylation originates from the characterization of mucoproteins and mucopolysaccharides. In 1835, people first used the term ‘mucin’ to describe substances separated from mucus. And then in 1865, it was recognized that mucin was a combination of protein and glycans. Thereafter, researchers performed many of the classical experiments in the late 1800s that established glycans as major constituents of mucins. In the early 1900s, two related discoveries on the agglutination of erythrocytes, one was the seminal discovery of the ABO blood groups in human serum, and the other was the characterization of influenza virus hemagglutination, promoted the research of mucins. To date, over 150 mucin-type O-linked glycoproteins have been annotated in the SWISS-protein database, which are involved in a variety of biological processes.
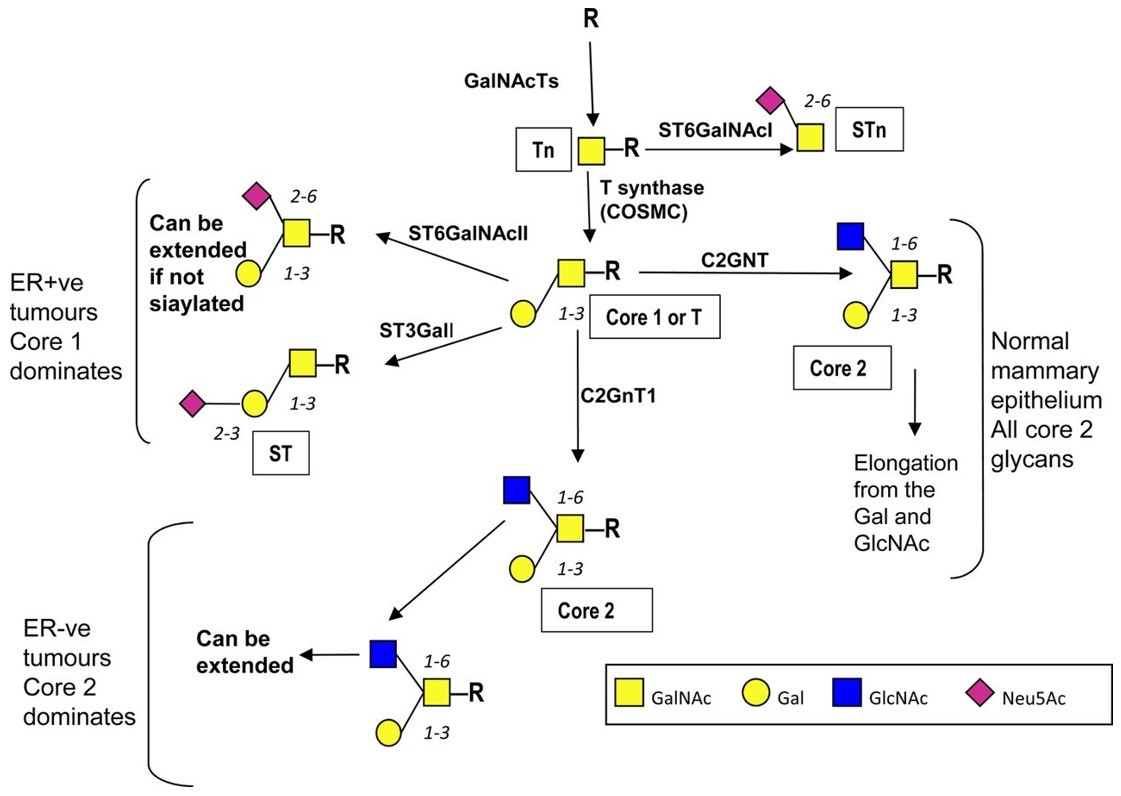 Fig.1 Pathways of mucin-type O-linked glycosylation in the normal and malignant mammary gland.1, 2
Fig.1 Pathways of mucin-type O-linked glycosylation in the normal and malignant mammary gland.1, 2
The Function of O-linked Glycoproteins
The physicochemical properties of glycoproteins can be changed by changing the glycan content. Sulfated or Sialylated glycans change the overall charge and increase the solubility of a glycoprotein, essentially for the highly sulfated proteoglycans and highly glycosylated mucins. They are found in mucous secretions of many epithelial cells and provide a gelation function due to their capability to retain water. They protect the epithelial boundaries by functioning as lubricants and mediating transport.
O-linked glycoproteins play a major role in defense against pathogenic microorganisms. Carbohydrate-specific adhesins on bacterial fimbriae can specifically interact with O-linked glycans on epithelial mucins and that such multifunctional binding in the mucus layer should entrap the pathogen and prevent infection. Evidence for specific lectin-carbohydrate interactions has been revealed for S-fimbriated E. coli and MUC1-bound sialic acid and for rotavirus replication, which is inhibited by the milk mucin. Therefore, O-linked glycoproteins are regarded as one of the initial barriers belonging to the components of innate immunity.
Services at Creative Biolabs
The dense glycosylation in the tandem repeats of mucins enables them to function as protective barriers and provide lubrication in a variety of tissues due to their hydration capacity. At present, many companies have learned from the characteristics of mucin to develop innovative drugs, such as medical adhesives, skin tissue repair agents, antibacterial drugs, and so on.
As a world-class provider of biotechnology services, we are committed to providing customers with Glycoengineering Service in the drug development process, such as Glyco-engineered Mammalian Cell Expression System, Glyco-engineered Pichia pastoris Expression System, Glyco-engineered Plant-based Expression System, etc. If you have any questions about glycoengineering, please contact us for more information.
References
-
Burchell, Joy M., et al. "O-linked mucin-type glycosylation in breast cancer." Biochemical Society Transactions 46.4 (2018): 779-788.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Pathways of mucin-type O-linked glycosylation in the normal and malignant mammary gland.1, 2
Fig.1 Pathways of mucin-type O-linked glycosylation in the normal and malignant mammary gland.1, 2