Glycoprotein Function as Antifreeze Protein
There are several different types of biological antifreezes in nature. These compounds can be found in various fish, plants, amphibians, and insects and can be divided into two categories: antifreeze glycoproteins (AFGPs) and antifreeze proteins (AFPs).
What are AFGPs?
AFGPs are a new class of compounds with biological significance, which can inhibit ice growth in vivo and in vitro. Although AFGPs have been found in several fishes, they have great structural similarities. The concentration of AFGPs in vivo is 4 ~ 50 mg/ml. AFGPs are composed of 8 different glycopeptides with molecular weights ranging from 2.6 to 34 kDa. Generally, AFGP between 20 ~ 33 kDa is recorded as AFGP 1 ~ 4, and those less than 20 kDa are recorded as AFGP 5 ~ 8, of which AFGP 8 is the smallest.
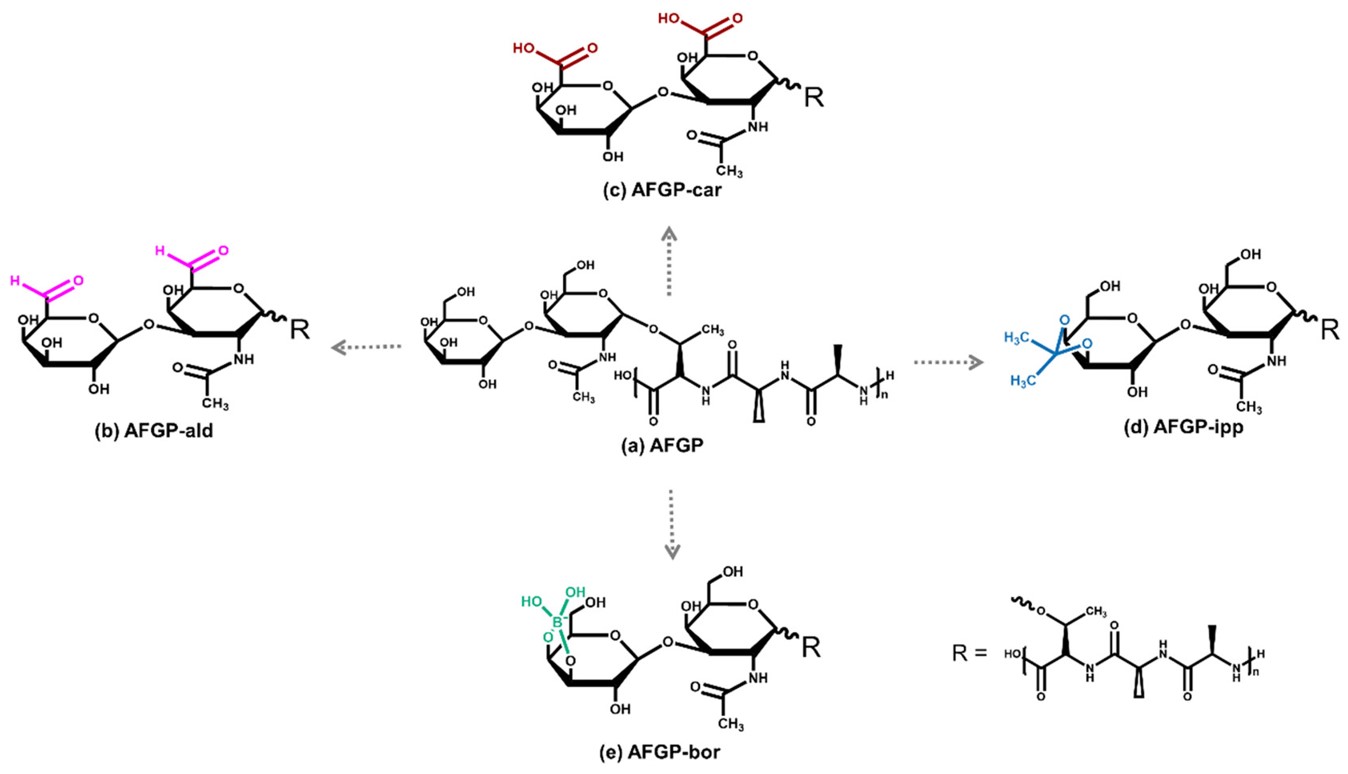 Fig.1 Structure of the natural AFGP and four modified variants.1, 3
Fig.1 Structure of the natural AFGP and four modified variants.1, 3
Custom Services for Glycoprotein Research
In the past decade, antifreeze glycoproteins have shown great potential in many industrial, commercial, and medical applications. At present, the only way to obtain natural AFGP is to isolate and purify it from deep-sea polar fish, which is time-consuming and expensive. Unfortunately, this is not suitable for large-scale production and commercial applications. Therefore, the chemical synthesis of AFGP and AFGP analogues is an attractive choice.
AFGPs are presently only available from natural sources in limited amounts. Hence recent research has focused on the development of an efficient synthetic route to AFGPs and analogues. The synthesis of glycoproteins and carbohydrates is significantly more demanding than protein synthesis. While automated solid-phase peptide synthesis or molecular biology techniques allow the routine production of AFPs, as well as the incorporation of mutations and isotopic labels into AFP sequences.
The first and only synthesis of a naturally occurring AFGP was reported in 1996 and is summarized in Fig. 2. An alternate route to high molecular mass AFGPs using solid-phase peptide synthesis has recently been reported using Fmoc-chemistry and standard protecting groups to produce AFGPs where n=4 and 8. The advantage of using solid-phase synthesis is the ability to generate oligomers of defined length and sequence variation, including mutations of the Ala residues at one or more sites in the sequence and modification of the structure of each sugar by the preparation of a different Fmoc-protected building block. In contrast, the solution phase route will always produce mixtures of oligomers that need to be separated, and require the use of a single tripeptide unit for the polymerization reaction.
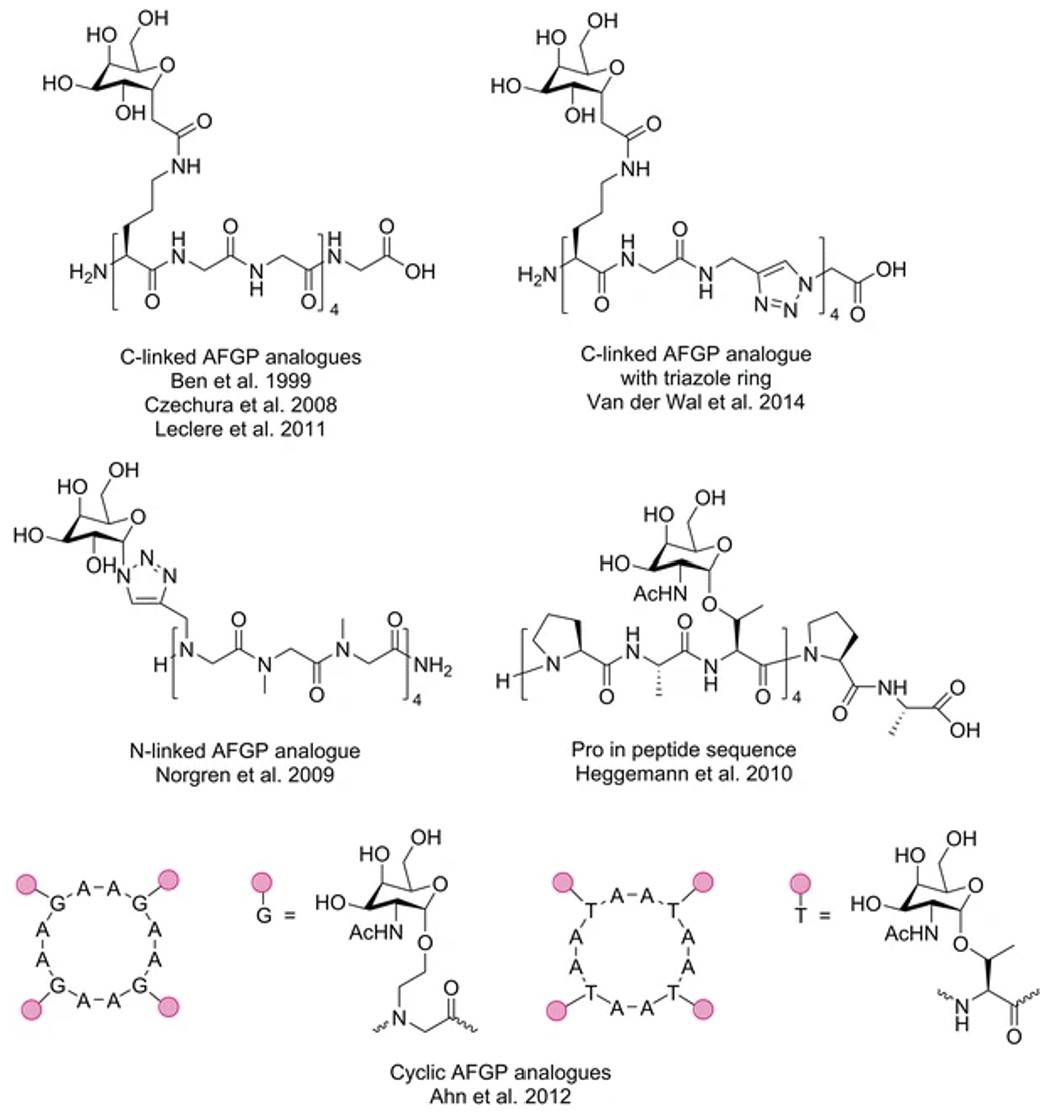 Fig.2 Synthesized AFGP analogs.2, 3
Fig.2 Synthesized AFGP analogs.2, 3
Applications
Both AFGPs and AFPs exhibit some unique properties which protect biological systems in vitro and have been investigated for potential applications in medicine, biotechnology, and the food industry. The ability to change the normal growth habit of ice, the capacity to inhibit recrystallization and the protection of cell membranes are all properties of AFPs and AFGPs that may be tailored for a range of low temperature processes. The ability of AFGPs to aid in the cryopreservation and hypothermal storage of cells and tissues. Scientists observed that AFGP and AFP possessed the ability to enhance external cell membrane integrity during cooling and protect against rupture. They suggested that membrane integrity was enhanced because AFGP blocked ion fluxes across the membrane. AFGPs and AFPs have been identified as useful in cryosurgery, increasing the destruction of solid tumors through mechanical damage to cells caused by the growth of bipyramidal ice crystals. Both AFPs and AFGPs have attracted significant interest as potential food additives that inhibit ice recrystallization and hence the formation of large ice crystals in the storage of frozen foods.
Creative Biolabs is a biotechnology company specializing in glycoprotein research. With the equipment, knowledge, and technology, we can provide customized services and advanced technology for your glycoprotein research. We can specify the most reasonable solution for you, and promote your project more efficiently. If you are interested in our services or technology, please contact us for more details.
References
-
Sun, Yuling, et al. "Disaccharide residues are required for native antifreeze glycoprotein activity." Biomacromolecules 22.6 (2021): 2595-2603.
-
Urbańczyk, Małgorzata, et al. "Antifreeze glycopeptides: from structure and activity studies to current approaches in chemical synthesis." Amino Acids 49 (2017): 209-222.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Structure of the natural AFGP and four modified variants.1, 3
Fig.1 Structure of the natural AFGP and four modified variants.1, 3
 Fig.2 Synthesized AFGP analogs.2, 3
Fig.2 Synthesized AFGP analogs.2, 3

