Glycoprotein Crystal based Glycosylation Type Analysis Service
Introduction
Are you currently facing low-resolution crystal structures for glycosylated targets, heterogeneous protein samples, and stalled lead optimization efforts? Our One-Stop Glycan Crystal & Glycoprotein Crystal Analysis Services help you achieve atomic-level resolution and define Glycosylation Sites critical for ligand binding through the proprietary technology platform combined with high-throughput X-ray crystallography and mass spectrometry (MS) analysis. Glycosylation is a ubiquitous post-translational modification (PTM) critical for protein function, especially in cell-surface receptors. The inherent heterogeneity of N-glycans, however, poses a major barrier to high-resolution structural studies. Our service addresses this by providing precise structural mapping of these glycans, enabling the rational design of therapeutics that target these PTM-dependent interactions and successfully overcoming the limitations associated with conventional recombinant protein production.
Structural biology of glycoproteins is the necessary precursor to rational drug design, but the inherent heterogeneity of N-glycans frequently acts as a resolution barrier. Our service provides the validated, high-resolution structural coordinates required to map protein-glycan and critical glycan-glycan interactions, which directly inform the development of specific therapeutic agents. We deliver a clear, actionable blueprint for your lead compounds.
|
Stage
|
Key Steps Involved
|
Expected Outcomes
|
|
Required starting materials
|
Target gene sequence, preferred expression construct, and existing low-resolution structural or biophysical data (if available).
|
An initial assessment of the protein's complexity and project scope.
|
|
Glyco-engineered expression
|
Cloning and expression of the target protein into our proprietary cell line, which eliminates complex, heterogeneous glycans.
|
High yields of soluble, homogeneous glycoprotein suitable for crystallogenesis, verified by SDS-PAGE and initial MS.
|
|
Crystallogenesis and data collection
|
High-throughput screening of crystallization conditions, optimization of crystal contacts, and collection of high-resolution X-ray diffraction data at synchrotron sources.
|
Diffraction-quality crystals, raw X-ray data sets, and preliminary data processing statistics showing high completeness.
|
|
Atomic structure determination
|
Solving the phase problem via molecular replacement, iterative model building in electron density maps, and detailed refinement.
|
Obtain a high-resolution data file.
|
|
Glycan and structural analysis
|
Post-crystallization MS analysis of the crystallized protein to confirm the specific glycan composition and occupancy at each N-linked site. Structural analysis is performed to characterize the binding interface and oligomeric state (monomer, dimer, hexamer, etc.).
|
Obtain the oligomeric state and precise coordinates of glycan moieties.
|
The comprehensive glycoprotein crystal based glycosylation type analysis service is meticulously structured to ensure a successful outcome, typically ranging from 12 to 20 weeks, depending on the target's complexity and stability challenges.

Challenges and Necessity in Glycoprotein Structural Analysis
The core challenge in glycoprotein structural analysis is glycan heterogeneity. Standard mammalian expression systems produce a mix of complex and hybrid glycans of varying lengths (antennae), which introduces conformational flexibility on the protein surface. This flexibility directly hinders the formation of the highly ordered crystal lattice required for X-ray diffraction. Furthermore, even if crystals are obtained, the resulting disorder of the flexible glycans often limits the resolution to worse, making structural determination unreliable and omitting crucial details about glycan-protein interactions. Precise knowledge of glycosylation type and structure is necessary because N-glycans frequently regulate receptor functions, stabilize structure, and define cell-cell recognition. High-resolution structural data combined with analytical confirmation (MS) is the way to definitively characterize these functional PTMs and move forward with rational drug design.
Why Choose Us?
Creative Biolabs is recognized for integrating structural biology expertise with deep knowledge of glycochemistry. Our commitment to high resolution and accuracy provides reliable data that accelerates your research.

Atomic precision in glycan modeling
We specialize in interpreting the often-weak and ambiguous electron density associated with flexible glycans, ensuring the most accurate model possible for the fixed core residues.

Integrated multi-omic perspective
While X-ray analysis provides 3D context, we combine this structural insight with an understanding of MS data (often provided by the client or through our related services) to build the most biologically relevant model of the glycan types present.

Rigorous validation standards
All structures are refined and validated using industry-standard metrics to ensure the highest quality model.
Published Data
The structural analysis of glycoproteins using X-ray crystallography is frequently complicated by the presence of highly diverse and branched sugar chains, or N-glycans, which interfere with the close, ordered packing required for crystal formation. To overcome this limitation, the authors leveraged the GlycoDelete (GD) HEK293 cell line, which is genetically modified to produce proteins carrying simplified, homogeneous N-glycan stubs, typically consisting of only three sugar units. This strategy was highly effective for heavily glycosylated proteins like fragments of the human Down syndrome cell-adhesion molecule (DSCAM) and the colony-stimulating factor 1 receptor (CSF-1R). Production in GD cells resulted in protein preparations with dramatically increased homogeneity and reduced molecular weight, enabling the successful growth of high-quality crystals that diffracted to resolutions necessary for structure determination, a result unattainable with standard cell lines.
The study also confirmed the system's flexibility by testing murine IL-12B. As clearly illustrated in the following Figure, the resulting crystal structure did not display the expected simplified GD trisaccharide. Instead, the IL-12B protein retained an immature high-mannose type glycan (specifically Man4GlcNAc2) at the Asn220 position. This suggests that the immature glycan, which is structurally shielded and likely critical for the protein's proper folding and stability, avoids the simplification machinery within the GD cell line. Thus, the GD cell line serves as an exceptional tool for structural biology, as it uniformly truncates non-essential, flexible glycans to promote crystallogenesis while preserving specific, functionally or structurally required immature sugar moieties.
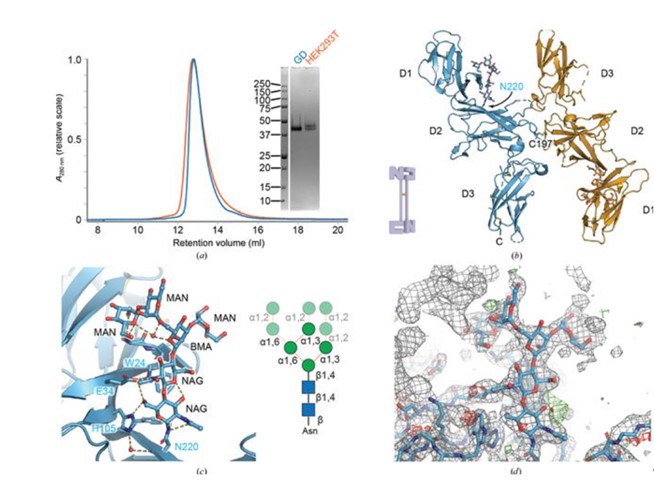 Fig.1 High-resolution crystal structure of IL-12B revealed.1
Fig.1 High-resolution crystal structure of IL-12B revealed.1
FAQs
Is the combination of crystallography and MS always necessary?
We strongly recommend it. MS provides a complete inventory of all the different glycan structures present on your protein (microheterogeneity), but cannot tell you where they are in 3D space. Crystallography reveals where the glycan is. This combined validation minimizes the risk of basing critical drug design decisions on an unvalidated structural model. Our service provides the atomic-level 3D coordinates of the most ordered glycan core at a specific site, showing its exact interaction with the protein. We recommend using both MS and our structural analysis for the most comprehensive characterization.
Why is standard commercial protein expression insufficient for crystallization?
Standard systems produce glycoproteins with high PTM heterogeneity (a mix of complex, hybrid, and high-mannose glycans). This mixture acts as a blend of different molecules, preventing the organized packing required for crystallization. Our service uses engineered cell lines to achieve the necessary molecular uniformity.
My glycoprotein has very heterogeneous glycosylation. Can your service still provide useful data?
Absolutely. Glycan heterogeneity is the primary challenge in structural glycobiology. We often employ specific enzymatic trimming steps to reduce the heterogeneity to a simple, stable core. This core structure alone provides vital information about the site of attachment and the steric footprint of the glycan.
Customer Review
Unprecedented Clarity
"Using Creative Biolabs' glycoprotein crystal based glycosylation type analysis service in our research has significantly improved our understanding of how N-glycans facilitate stabilizing crystal contacts, leading to high-resolution data on a challenging adhesion molecule. Highly recommended for complex glycoproteins."- Dr. K**l.
Rational Design Validated
"Creative Biolabs' comprehensive report clearly mapped the dimeric interface of our target. This structural certainty allowed us to rapidly pivot our lead molecule design to block the inhibitory site directly, based on the flexible hydrophobic core structure. This level of detail is impossible with lower resolution methods."- Prof. W***g.
Contact Our Team for More Information
Creative Biolabs provides the highest-resolution Glycoprotein Crystal Analysis solutions for complex glycoprotein targets in the biopharmaceutical industry. By integrating the innovative expression platform with advanced X-ray crystallography and confirming the results with MS, we eliminate the structural uncertainty caused by glycan heterogeneity, allowing you to move confidently into lead optimization. Please contact us to accelerate your drug discovery process!
Reference
-
Kozak, Sandra, et al. "Homogeneously N-glycosylated proteins derived from the GlycoDelete HEK293 cell line enable diffraction-quality crystallogenesis." Biological Crystallography 76.12 (2020): 1244-1255. Distributed under an Open Access license CC BY 4.0, without modification. https://doi.org/10.1107/S2059798320013753
For Research Use Only.
Related Services





 Fig.1 High-resolution crystal structure of IL-12B revealed.1
Fig.1 High-resolution crystal structure of IL-12B revealed.1

