Sugar based Intermediate Synthesis Service
The Glycochemical Revolution: Integrated Sugar Intermediate Synthesis
Are you currently facing challenges in synthesizing complex, stereo-specific pharmaceutical intermediates, difficulty in achieving drug solubility and bioavailability, or a lack of sustainable synthesis routes? Our sugar based intermediate synthesis service helps you de-risk drug candidates and streamline manufacturing processes through an integrated platform combining advanced biocatalysis, chemocatalysis, and supramolecular design. The field of glycochemistry is central to modern pharmaceutical science, as carbohydrates are key components of cell recognition, therapeutic activity, and drug delivery systems. Our sugar based intermediate synthesis service provides access to the structural diversity of the glycome, moving beyond simple commodity sugars to offer customized, high-purity intermediates. This service is grounded in the strategic convergence of sustainable feedstock utilization, selective enzymatic synthesis, and efficient catalytic scaling. Creative Biolabs enables the creation of highly specialized molecules that confer improved targeting, stability, and therapeutic efficacy to your final drug product.
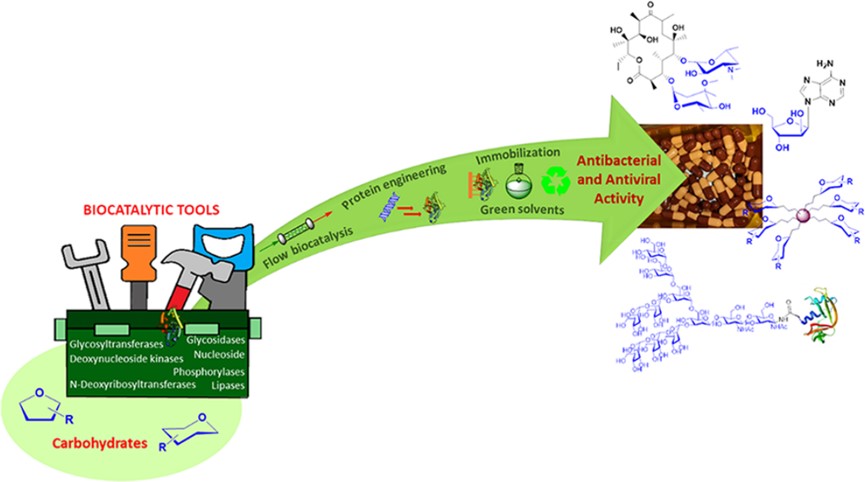 Fig.1 Carbohydrates play an important role in living systems.1
Fig.1 Carbohydrates play an important role in living systems.1
Creative Biolabs provides highly customized sugar-based intermediates that solve critical formulation and targeting challenges. We deliver compounds with unmatched stereochemical purity and offer flexible scale-up options, from milligram research quantities to kilogram process development lots. Our unique capability to synthesize complex, high-value rare sugars and scalable Custom Synthesis Services directly translates to enhanced drug efficacy and superior manufacturing economics for your therapeutic project. Creative Biolabs' service addresses the fundamental need for high-quality, complex carbohydrate structures and their derivatives. This detailed workflow provides a transparent overview of the integrated process that underpins our intermediate synthesis service, designed for seamless collaboration and clear outcomes.
|
Phase
|
Key Steps Involved
|
Description of Activities & Expected Outcomes
|
|
I. Intake & Strategy
|
Required starting materials
|
We require the target structure/formula of the desired intermediate, the current synthetic route (if available), and the intended application (e.g., prodrug, linker, excipient, API).
|
|
II. Route Design
|
Biocatalytic & chemocatalytic pathway selection
|
Based on complexity and scale requirements, we design the optimal synthesis route: biocatalysis (for high-selectivity, complex rare sugar motifs) or chemocatalysis.
|
|
III. Synthesis & Optimization
|
Process development & scale-up
|
This involves lab-scale synthesis, reaction condition optimization (temperature, solvent, catalyst type), and purification method development.
|
|
IV. Validation
|
Quality control & final analysis
|
Comprehensive structural validation using state-of-the-art analytical techniques (NMR, Mass Spectrometry, HPLC purity). This phase confirms the stereochemical fidelity and purity of the final product.
|
|
V. Delivery
|
Final deliverables
|
Product delivery, comprehensive process report, and safety data sheets (SDS). These deliverables ensure a seamless transition for your team, providing the pure intermediate and the necessary data.
|
Creative Biolabs utilizes a highly adaptable strategy based on the final application, scale, and complexity of the target molecule. We utilize our proprietary enzyme library and biocatalytic expertise for maximum structural fidelity.
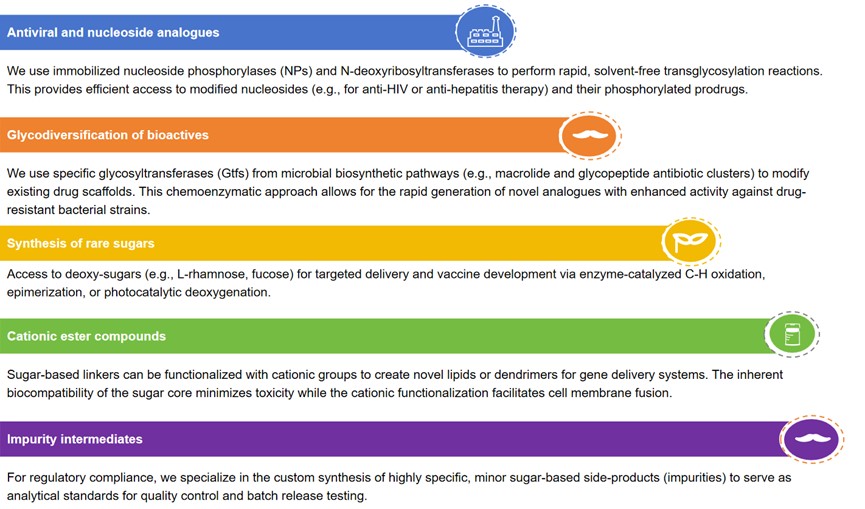
Why Sugar-Based Intermediate Synthesis Services Are Necessary
Carbohydrates are information-rich molecules that determine the fate of a therapeutic agent in the body. Creative Biolabs is not just a synthesis house; we are a strategic partner providing integrated glycochemical solutions that drive measurable success. Our expertise spans the entire synthesis spectrum, allowing us to select the most cost-effective and highest-purity pathway for your project.
-
Chirality and stereoselectivity: Sugar molecules are highly chiral, meaning a slight difference in stereochemistry can completely change biological activity (e.g., from therapeutic to toxic). Enzymes offer precise control that chemical synthesis struggles to replicate.
-
Targeting and signaling: Sugars serve as biological recognition motifs (epitopes). Incorporating specific rare sugars allows for active targeting, guiding the drug to specific cell receptors or disrupting microbial communication.
-
Sustainability and green chemistry: Sourcing from abundant, renewable materials like cellulose and glucose reduces reliance on petroleum-derived starting materials, aligning your pipeline with global sustainability mandates.
Published Data
Biocatalysis serves as a sustainable and efficient method for synthesizing complex glycostructures, which are essential for combating bacterial and viral infections, thereby moving away from environmentally taxing conventional chemical processes. The central concept, categorized into different strategic pillars, demonstrates how specific families of enzymes provide superior chemo-, regio-, and stereoselectivity for pharmaceutical production. First, the synthesis of antiviral nucleoside and nucleotide analogues leverages enzymes such as nucleoside phosphorylases (NPs) and deoxynucleoside kinases (DNKs). These biocatalysts facilitate highly efficient transglycosylation and selective phosphorylation reactions, often in aqueous or flow systems, to produce therapeutic agents for diseases such as HIV and herpes. Second, the preparation of large glycoconjugates employs lipases, glycosidases, and glycosyltransferases (Gtfs). These enzymes are key to creating pure, structure-defined antigenic oligosaccharides and their subsequent conjugation to protein or dendrimer scaffolds. Third, the study highlights the use of specialized glycosyltransferases sourced from the natural biosynthetic pathways of antibiotics (like macrolides and glycopeptides). This approach is instrumental in the chemoenzymatic modification of existing antibiotic scaffolds, allowing researchers to generate novel derivatives with improved potency and the ability to counter mounting antibiotic resistance. Ultimately, the review concludes that the combination of these specialized enzymatic tools with advanced techniques like immobilization and green solvents offers an indispensable, environmentally responsible toolbox for accelerating drug discovery in glycosciences.
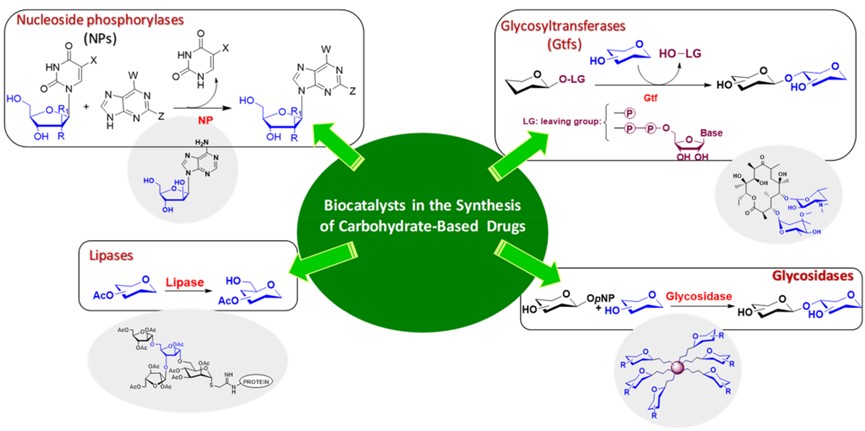 Fig.2 Sugar-based drug synthesis strategies.1
Fig.2 Sugar-based drug synthesis strategies.1
FAQs
My API is highly hydrophobic. How can your service guarantee improved solubility compared to standard excipients?
Our approach goes beyond standard excipients. We specialize in synthesizing advanced sucrose-based macrocycles. These function as "host-guest" systems, where the API is stoichiometrically encapsulated within a highly water-soluble molecular cage, leading to predictable and superior solubility and bioavailability compared to traditional polymeric formulations.
We need to modify a complex antibiotic. Why should we choose your biocatalytic method over standard chemical glycorandomization?
Standard chemical modification is non-selective, leading to purification challenges. Our chemoenzymatic approach uses purified glycosyltransferases to achieve site-specific, stereoselective modification of the sugar moiety. This ensures high purity and better yield. Discuss your target structure with our expert chemists today.
What materials do you need to start, and what is the typical turnaround for a novel rare sugar intermediate?
To start, we need the target structure, desired quantity, and purity specifications. For a novel rare sugar that requires complex enzymatic or photocatalytic route development, the process typically takes 12 to 20 weeks to deliver the fully characterized product and detailed process report. We can provide a precise project timeline after a brief consultation.
Customer Review
Tangible Cost Savings
"Using Creative Biolabs' synthesis service in our API modification research has significantly improved the cost-efficiency of our supply chain. Their ability to leverage inexpensive, chemocatalytic routes for C6 blocks like Isosorbide means we no longer rely on expensive, low-yield multi-step chemical syntheses for our core excipients."- Dr. Ar**Js, Project director.
Purity in Complex Sugars
"The stereochemical fidelity of the rare sugar library supplied by Creative Biolabs is exceptional. This directly facilitated our high-throughput screening of novel antibacterial agents, as we could confidently link biological activity to a single, pure stereoisomer, significantly streamlining SAR studies and avoiding false positives."- Ma**Kn, Scientist.
How to Contact Us
Creative Biolabs provides an integrated solution for complex sugar based intermediate synthesis service, ensuring your therapeutic leads benefit from molecular precision, sustainable sourcing, and scalable production. From complex rare sugar targeting ligands created via biocatalysis to commercial platform excipients produced via chemocatalytic flow, we are your strategic partner in glycochemical innovation. Ready to advance your drug candidate with Creative Biolabs' expertise? Please contact us for more information.
Reference
-
Hoyos, Pilar, et al. "Biocatalyzed synthesis of glycostructures with anti-infective activity." Accounts of Chemical Research 55.17 (2022): 2409-2424. Distributed under an Open Access license CC BY 4.0, without modification. https://doi.org/10.1021/acs.accounts.2c00136
For Research Use Only.
Related Services

 Fig.1 Carbohydrates play an important role in living systems.1
Fig.1 Carbohydrates play an important role in living systems.1

 Fig.2 Sugar-based drug synthesis strategies.1
Fig.2 Sugar-based drug synthesis strategies.1

