Exchanging Larger Amino Acid Stretche Between Different Isotypes
Creative Biolabs empowers global research by precisely engineering antibody frameworks through strategic isotype exchange and larger domain swaps. We tackle complex biological challenges, optimize protein function with meticulous accuracy, and enhance therapeutic efficacy, accelerating our clients' discoveries.
Background
Antibodies, particularly IgG, are essential for the adaptive immune system, binding and neutralizing pathogens. Their therapeutic potential is enhanced by structural modifications, and while understanding antibody structure is fundamental to engineering effective therapeutics, a key advancement is modifying the Fc region to fine-tune effector functions. Traditional IgG antibodies don't interact with the FcαRI receptor on myeloid cells, but IgA antibodies can elicit potent cytotoxic action via FcαRI. Since there are challenges with IgA expression and purification for therapeutic use, there's a need to combine the advantages of different isotypes. Exchanging Larger Amino Acid Stretches Between Different Isotypes at Creative Biolabs offers a solution by creating 'cross-isotype' antibodies, like IgGA, that bind to both FcαRI (like IgA) and FcγRI/FcγRIIa (like IgG1), enhancing their therapeutic potential and improving therapeutic outcomes.
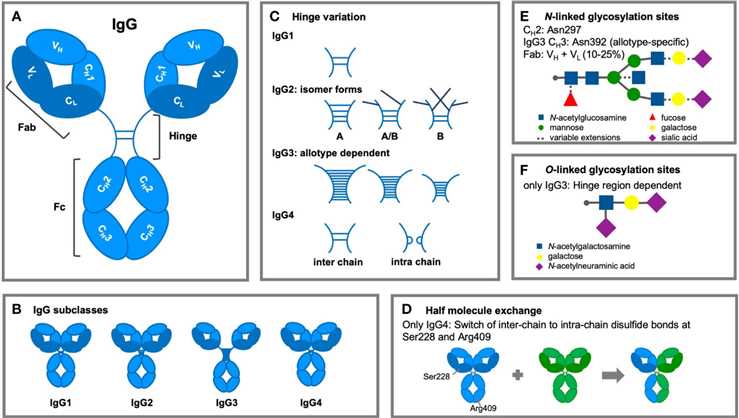 Fig.1 Improvements in the structure of IgG antibodies.1
Fig.1 Improvements in the structure of IgG antibodies.1
Exchanging Larger Amino Acid Stretches Between Different Isotypes at Creative Biolabs
Creative Biolabs' service enables the development of therapeutic antibodies with optimized effector functions by exchanging larger amino acid sequences between different antibody isotypes. This approach allows you to tailor antibody properties to specific therapeutic needs. This capability is particularly valuable in the development of next-generation antibody therapeutics, where fine-tuning the antibody's interaction with the immune system can significantly impact its efficacy and safety.
End-to-End Service Workflow
At Creative Biolabs, our exchanging larger amino acid stretches between different isotypes service involves a structured process to ensure the generation of optimized antibodies:
Sequence Analysis and Design: The provided antibody sequences are analyzed, and an optimal isotype exchange strategy is designed to achieve the desired effector functions. This process involves the identification of key regions within the Fc domain that are suitable for isotype exchange.
Construct Generation: Advanced gene synthesis and cloning techniques are employed to generate DNA constructs. These constructs encode the engineered antibody, which features the isotype-switched Fc region.
Expression and Purification: The engineered antibody is expressed in a suitable host system, such as mammalian cells. Subsequently, rigorous purification methods are utilized to obtain a highly pure antibody product.
Functional Characterization: The engineered antibody undergoes comprehensive in vitro functional characterization. This characterization includes assays designed to assess binding to Fc receptors (FcγRs, FcαRI), as well as the evaluation of effector functions, such as ADCC, ADCP, and CDC.
Delivery and Quality Control: To make sure the finished product satisfies the necessary requirements, it undergoes extensive quality control testing. A detailed report containing all experimental data and analyses will be provided upon completion of the service.
Achievable Cross-Isotype Antibodies
Creative Biolabs' exchanging larger amino acid stretches between different isotypes service can be used to create a variety of cross-isotype antibodies, including but not limited to:
- IgG-IgA hybrids
- IgG-IgM hybrids
- IgG-IgE hybrids
- IgG-IgD hybrids
- Other novel combinations
Highlight Features
- Achieve desired therapeutic outcomes by combining the strengths of different antibody isotypes.
- Create innovative antibody-based therapies with unique mechanisms of action.
- Increase the effectiveness of antibody-mediated cell killing through enhanced ADCC, ADCP, and CDC.
- Customized strategy to satisfy particular project needs and targeted antibody characteristics.
FAQs
-
Q1: How long does it usually take to complete an antibody isotype engineering project?
A1: Antibody isotype engineering projects typically span 8 to 16 weeks, a timeframe shaped by the project's specific intricacies. We prioritize close collaboration with our clients to define achievable timelines and ensure swift, effective delivery of results.
-
Q2: Do different amino acid pairs have different relative exchangeabilities in cross-isotype antibodies?
A2: Yes, different amino acid pairs can indeed have different relative exchangeabilities in cross-isotype antibodies. The unique structural and functional roles of different antibody isotypes mean that the tolerance and consequences of amino acid substitutions, and therefore the relative exchangeability of amino acid pairs, can vary significantly across isotypes. This is an important consideration in antibody engineering efforts aimed at optimizing therapeutic antibody properties.
Related Services
Creative Biolabs offers a comprehensive suite of antibody engineering services to support your therapeutic antibody development needs. In addition to exchanging larger amino acid stretches between different isotypes, we provide complementary services, including:
Creative Biolabs: Your Trustworthy Partner
Creative Biolabs offers a unique advantage in antibody isotype engineering. Our expertise in Fc engineering, combined with advanced protein engineering platforms, ensures the successful development of cross-isotype antibodies with enhanced therapeutic potency. We have a proven track record of delivering high-quality, customized solutions to meet the diverse needs of our clients. If you are interested in exchanging larger amino acid stretches between different isotypes, please feel free to contact us.
Reference
- Damelang, Timon, et al. "Impact of structural modifications of IgG antibodies on effector functions." Frontiers in Immunology 14 (2024): 1304365. Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only.
