Toxicology Study Services
Introduction
Toxicology studies are essential for evaluating the safety profile of new drugs, chemicals, or other substances before they are released into the market. These studies assess the potential harmful effects of a compound on biological systems, helping to identify toxicity risks and establish safe exposure limits. Toxicology research involves a comprehensive approach, including acute, subchronic, and chronic toxicity testing, genotoxicity, carcinogenicity, reproductive toxicity, and organ-specific toxicity. By analyzing the dose-response relationship, toxicology studies can predict adverse effects, guide dose determination, and ensure the safety of human exposure. In modern drug development, these studies utilize both in vivo and in vitro models, ranging from animal studies to cell-based assays, to evaluate the safety and potential risks of a drug. Toxicology studies play a critical role in ensuring compliance with regulatory requirements and preventing harmful side effects that could impact public health. Creative Biolabs offers a wide range of toxicology services, including safety pharmacology, acute and chronic toxicity testing, genotoxicity assays, reproductive and developmental toxicity evaluations, and organ toxicity assessments. Our advanced testing platforms help identify risks early, ensuring that drug candidates meet safety standards for clinical trials and regulatory approval.
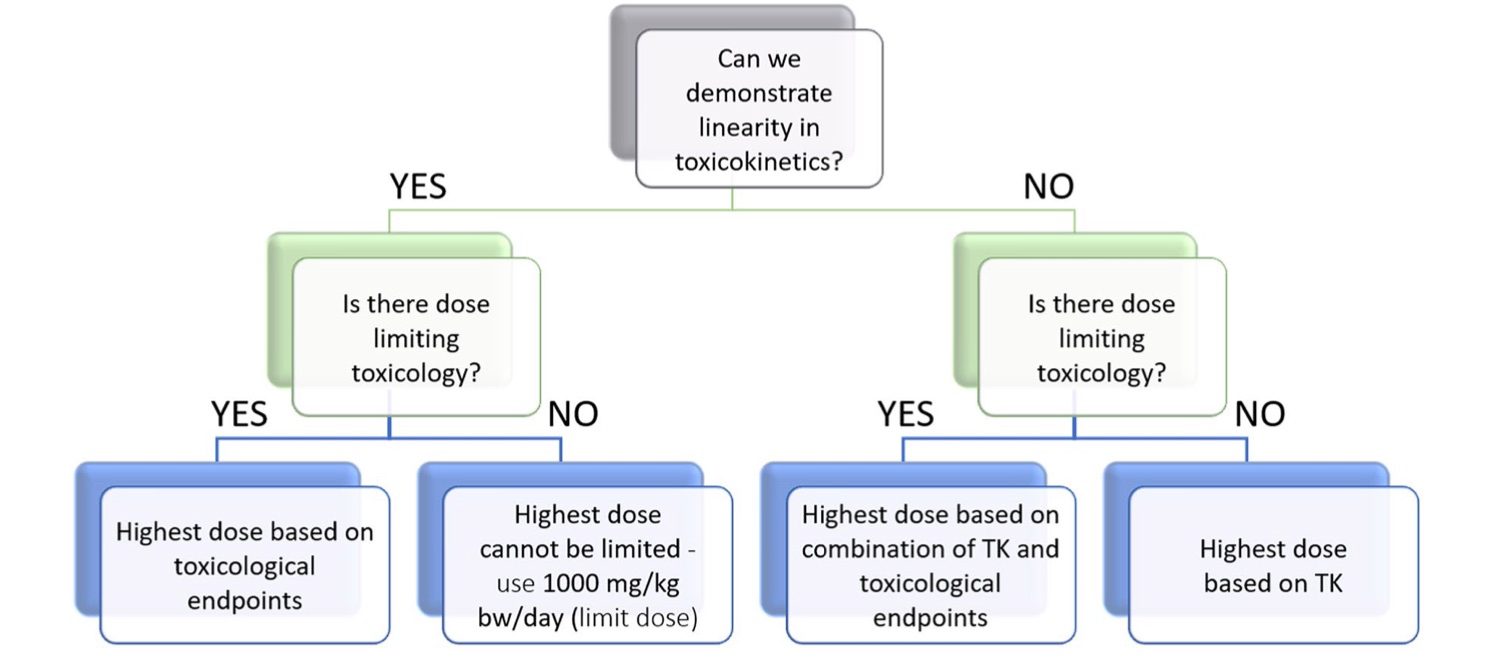 Fig. 1 Use of TK data in the design of toxicity studies.1
Fig. 1 Use of TK data in the design of toxicity studies.1
Our Services
In Vitro Toxicity Test
Our in vitro toxicity test services provide a reliable and efficient method for assessing the potential toxicity of compounds without the need for animal testing. Using advanced cell culture models, we evaluate cellular responses to chemical exposure, focusing on cytotoxicity, genotoxicity, and other toxicological endpoints. This approach enables early detection of adverse effects, helping to predict the safety profile of drug candidates, chemicals, or other substances. Our in vitro assays support regulatory compliance and aid in the development of safer, more effective products.
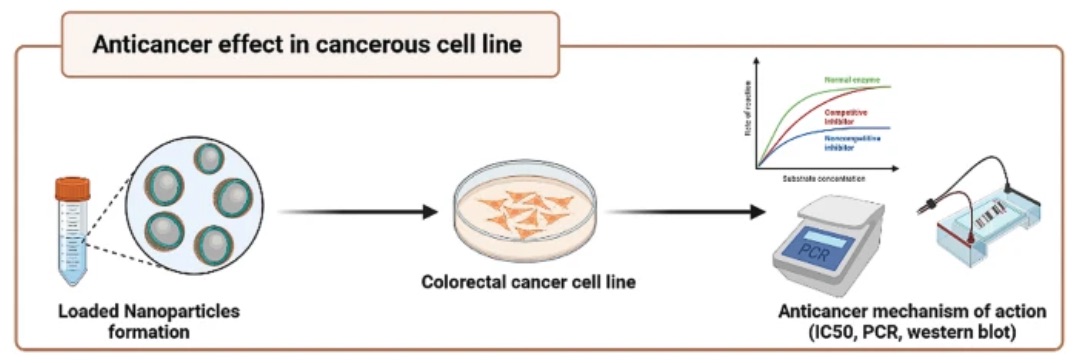 Fig. 2 The anticancer effect of a sericin/propolis/fluorouracil nanoformulation was evaluated in a cancerous cell line against colorectal cancer.2
Fig. 2 The anticancer effect of a sericin/propolis/fluorouracil nanoformulation was evaluated in a cancerous cell line against colorectal cancer.2
In Vitro Safety Pharmacology Studies
Our in vitro safety pharmacology studies focus on evaluating the potential pharmacological effects of drug candidates on critical biological systems, such as the cardiovascular, central nervous, and respiratory systems. Using advanced cell-based assays, we assess the safety profile of compounds by investigating their effects on key physiological functions, including ion channel activity, neurotransmitter release, and heart rate. These studies help identify potential risks early in the drug development process, ensuring that compounds are safe for further clinical testing and regulatory approval.
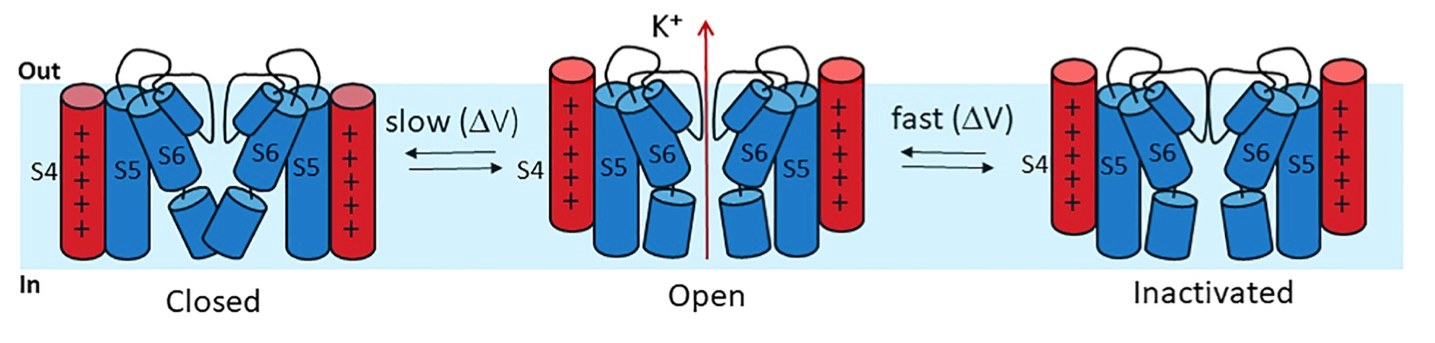 Fig. 3 Schematic of the hERG channel gating.3
Fig. 3 Schematic of the hERG channel gating.3
In Vivo Toxicity
Our in vivo toxicity services offer comprehensive testing to assess the potential harmful effects of substances in living organisms. Using animal models, we evaluate various toxicity endpoints, including acute, subchronic, and chronic toxicity, as well as organ-specific toxicity and genotoxicity. These studies help determine safe dosage ranges, identify possible adverse reactions, and guide the development of safer drug candidates or chemicals. Our in vivo testing is conducted in accordance with regulatory guidelines to ensure reliable data for clinical and commercial applications.
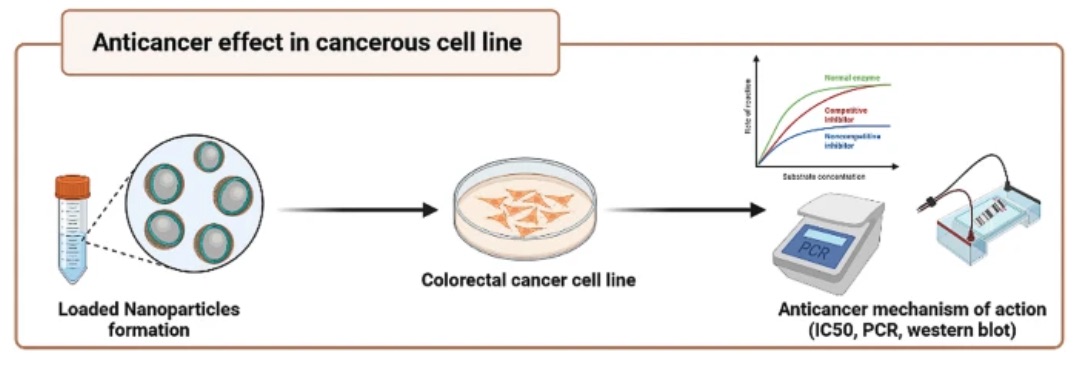 Fig. 4 The anticancer effect of a sericin/propolis/fluorouracil nanoformula was evaluated in an animal model against colorectal cancer.2
Fig. 4 The anticancer effect of a sericin/propolis/fluorouracil nanoformula was evaluated in an animal model against colorectal cancer.2
Platform
Our platform offers comprehensive toxicology study services using advanced methodologies to assess the safety profile of new drug candidates. These studies are conducted using a variety of animal models and cutting-edge instrumentation to provide reliable data for regulatory submissions and further clinical development.
1. Animal Models
-
Rodent Models
We use rat and mouse models for acute toxicity (single-dose exposure), sub-chronic toxicity (repeated-dose exposure), chronic toxicity (long-term exposure), and carcinogenicity (long-term cancer studies). These models are valuable for studying systemic toxicity and organ-specific damage, such as hepatotoxicity or nephrotoxicity. -
Rabbits
Rabbits are often used for local effects on skin/eyes (dermal/eye irritation), suitable for reproductive studies. -
Dogs
Dogs are good for repeated-dose studies and widely used in pharmaceutical toxicity. -
Non-Human Primate Models
In some cases, non-human primates are employed to study the pharmacokinetics, immunotoxicity, and neurotoxicity of drugs, providing a more translatable model for human exposure.
2. Instruments and Technologies
-
Histology & Imaging
We utilize advanced histological techniques, including H&E staining, immunohistochemistry (IHC), and Sirius Red staining for fibrosis, to assess tissue damage at the cellular level. -
High-Throughput Screening (HTS)
Our HTS systems provide rapid, large-scale testing for cytotoxicity, enabling high-throughput screening of numerous compounds under various conditions. -
Mass Spectrometry
Used for pharmacokinetic and metabolite analysis, allowing detailed profiling of drug absorption, distribution, and excretion pathways. -
Flow Cytometry
This technique helps quantify cellular responses such as apoptosis and cytokine production, especially in immune cells, to evaluate the immunotoxic effects of compounds.
Applications
Toxicology studies are vital for assessing the safety, efficacy, and potential adverse effects of chemical compounds, including pharmaceuticals, environmental toxins, and industrial chemicals. These studies have broad applications across multiple sectors, including drug development, environmental science, regulatory compliance, and public health.
Our Advantages
- Comprehensive Service Offerings: We provide a full spectrum of toxicology services, ranging from in vitro assays to in vivo animal studies. Whether you're assessing acute, chronic, or sub-chronic toxicity, our services cover a wide range of endpoints such as organ-specific toxicity, immunotoxicity, genotoxicity, and developmental toxicity.
- Experienced Team of Experts: Our toxicology studies are conducted by a team of highly skilled scientists with deep expertise in toxicology, pharmacology, and biomedical sciences. Our experts stay updated with the latest advancements in the field, ensuring that our studies meet regulatory guidelines and industry standards.
- State-of-the-Art Facilities and Instruments: We utilize cutting-edge instruments and technologies, including LC-MS/MS, confocal microscopy, flow cytometry, and automated histology scanning systems. These tools allow us to deliver high-quality, reproducible results, providing detailed insights into drug safety and toxicity.
- Customized Study Designs: Every project is unique, and we understand the importance of tailored study designs. Whether you need specific tests for a novel drug, environmental toxin, or consumer product, we design studies that meet your specific regulatory requirements and scientific objectives.
- Advanced Animal Models: We offer a wide variety of well-established animal models (rodents, non-human primates, fish, etc.) for evaluating drug toxicity. Our models are optimized to provide translatable data that predict human outcomes with high accuracy, ensuring the relevance and reliability of our findings.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1. How much do the studies cost?
A1: Cost depends on the study type and model used. We offer customized quotes based on your specific needs.
-
Q2. What kind of reports do you provide?
A2: We provide detailed reports with raw data, statistical analysis, study summaries, and regulatory-ready documentation.
-
Q3. Can you help with regulatory submissions?
A3: Yes, we provide comprehensive reports and documentation to support your regulatory submissions.
-
Q4: Can I get a custom study design?
A4: Yes, we offer customized study designs tailored to your specific needs and requirements.
References
- Sewell, Fiona et al. "Recommendations on dose level selection for repeat dose toxicity studies." Archives of toxicology vol. 96,7 (2022): 1921-1934. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1007/s00204-022-03293-3
- Diab, Shaimaa E et al. " In vitro and in vivo anti-colorectal cancer effect of the newly synthesized sericin/propolis/fluorouracil nanoplatform through modulation of PI3K/AKT/mTOR pathway." Scientific reports vol. 14,1 2433. 29 Jan. 2024. Distributed under Open Access license CC BY 4.0. The image was modified by extracting and using only part of the original image. https://doi.org/10.1038/s41598-024-52722-z
- Shi, Yu Patrick et al. "Modulation of hERG K+ Channel Deactivation by Voltage Sensor Relaxation." Frontiers in pharmacology vol. 11 139. 28 Feb. 2020. Distributed under Open Access license CC BY 4.0. The image was modified by extracting and using only part of the original image. https://doi.org/10.3389/fphar.2020.00139
For Research Use Only.
