Drug Metabolism & Pharmacokinetics (DMPK) Study Services
Introduction
Drug Metabolism and Pharmacokinetics (DMPK) is a critical discipline in drug development, focusing on understanding the absorption, distribution, metabolism, and excretion (ADME) of compounds within the body. These studies provide essential insights into how drugs interact with biological systems, determine their efficacy, and predict their safety profiles. DMPK studies are instrumental in optimizing drug formulations, dosing regimens, and overall therapeutic potential. By assessing parameters such as metabolic stability, half-life, bioavailability, and potential drug-drug interactions, DMPK helps in making informed decisions throughout the drug development process. In addition to ADME studies, anti-drug antibody (ADA) Services play a vital role in assessing the immune response to biologic drugs. ADA formation can impact the pharmacokinetics and overall efficacy of the drug, making it essential to monitor during development. With the aid of sophisticated analytical techniques like LC-MS/MS, DMPK studies can identify metabolic pathways, characterize metabolites, and predict the pharmacokinetic profile of novel drug candidates. This enables the early identification of potential liabilities such as poor bioavailability or toxic metabolites, which can be addressed before clinical trials. Creative Biolabs offers comprehensive DMPK services, including in vitro metabolism, pharmacokinetic studies, bioanalytical assays, drug-drug interaction assessments, and ADA testing. With advanced technologies and expertise, we help optimize drug candidates and support their progress through regulatory approval, ensuring both efficacy and safety for clinical use.
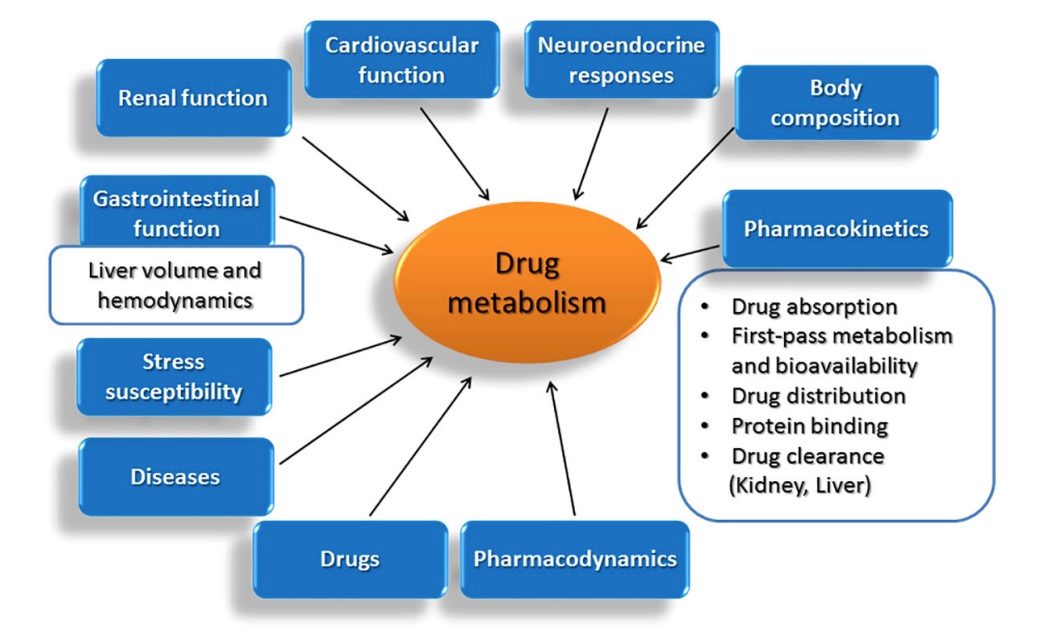 Fig.1 Factors that decisively influence the liver's drug metabolism capacity.1,5
Fig.1 Factors that decisively influence the liver's drug metabolism capacity.1,5
Our Services
In Vitro ADME Service
Our in vitro ADME service provides a comprehensive analysis of drug candidates to assess their pharmacokinetic properties. Through advanced in vitro testing, we evaluate key parameters such as permeability, metabolic stability, protein binding, and enzyme interactions, ensuring accurate prediction of drug behavior in the human body. This service supports early-stage drug development, helping to identify potential issues and optimize lead compounds for better efficacy and safety.
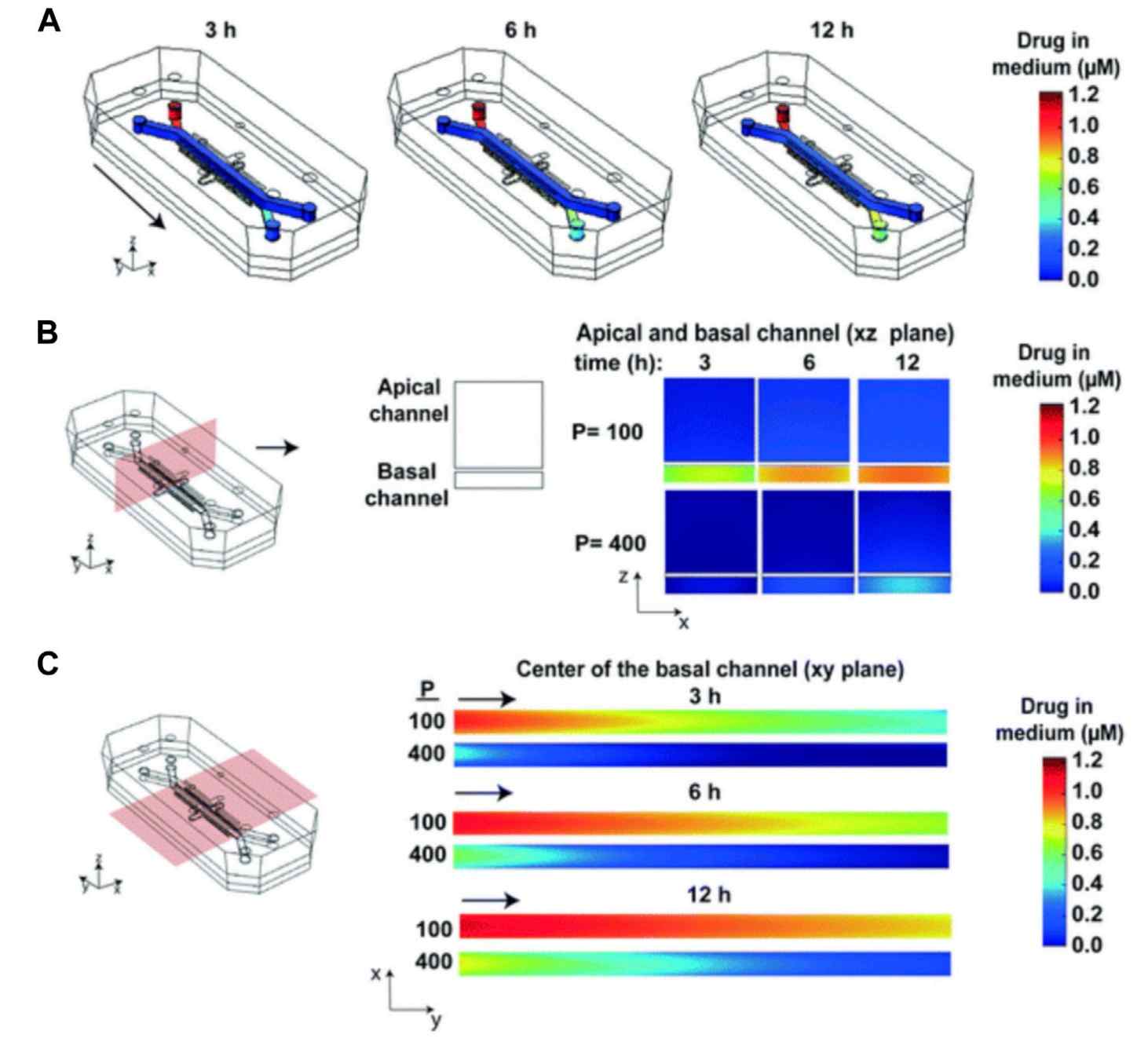 Fig. 2 Heat maps of the drug concentration.2,5
Fig. 2 Heat maps of the drug concentration.2,5
Anti-Drug Antibody (ADA) Services
Our anti-drug antibody (ADA) testing services, designed to assess the impact of immune responses on the effectiveness of treatments during drug development and clinical trials. ADA responses occur when the immune system produces antibodies against exogenous biologic drugs or small molecules, which may reduce the efficacy of the drug or trigger adverse reactions. Through precise ADA testing, we can early identify immune responses during drug development, optimizing treatment strategies and enhancing drug safety. Our services are customizable to meet the unique characteristics of different drugs, providing tailored ADA testing solutions to support a variety of drug development needs.
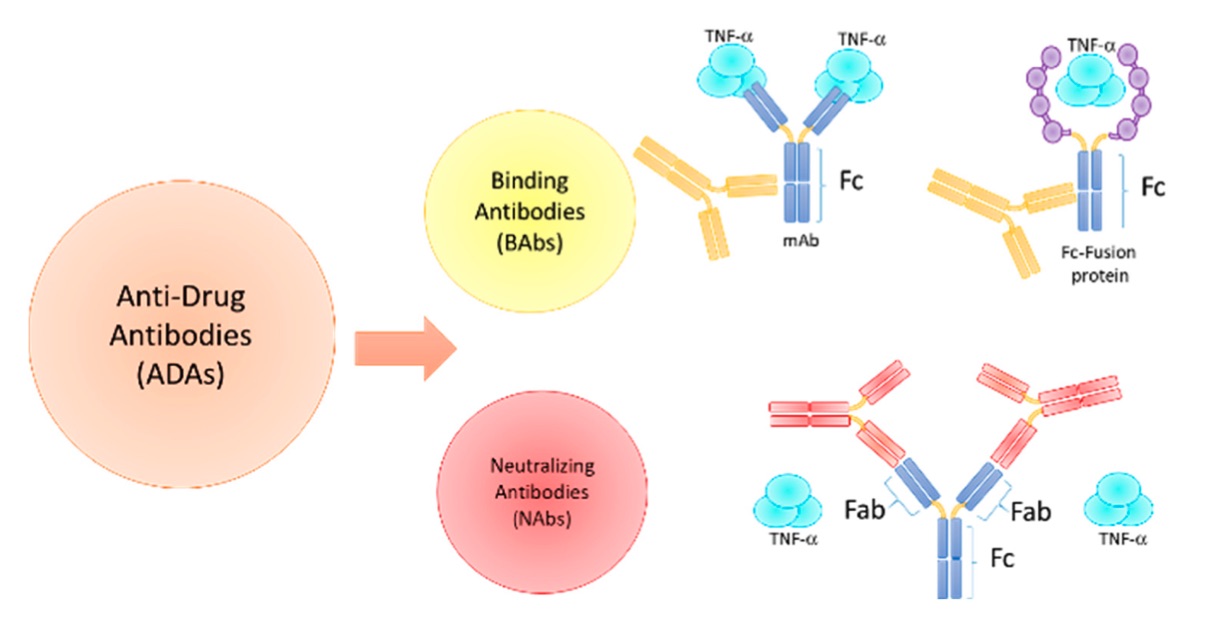 Fig.3 Anti-drug antibodies (ADAs).3,5
Fig.3 Anti-drug antibodies (ADAs).3,5
In Vivo PK Studies
Our in vivo ADME service offers a thorough assessment of drug absorption, distribution, metabolism, and excretion in living organisms. By utilizing animal models, we provide critical insights into the pharmacokinetic profile of drug candidates, including bioavailability, tissue distribution, and clearance rates. This service is essential for understanding drug behavior in complex biological systems and predicting human responses. Our team ensures accurate and reliable data, supporting informed decision-making in the drug development process.
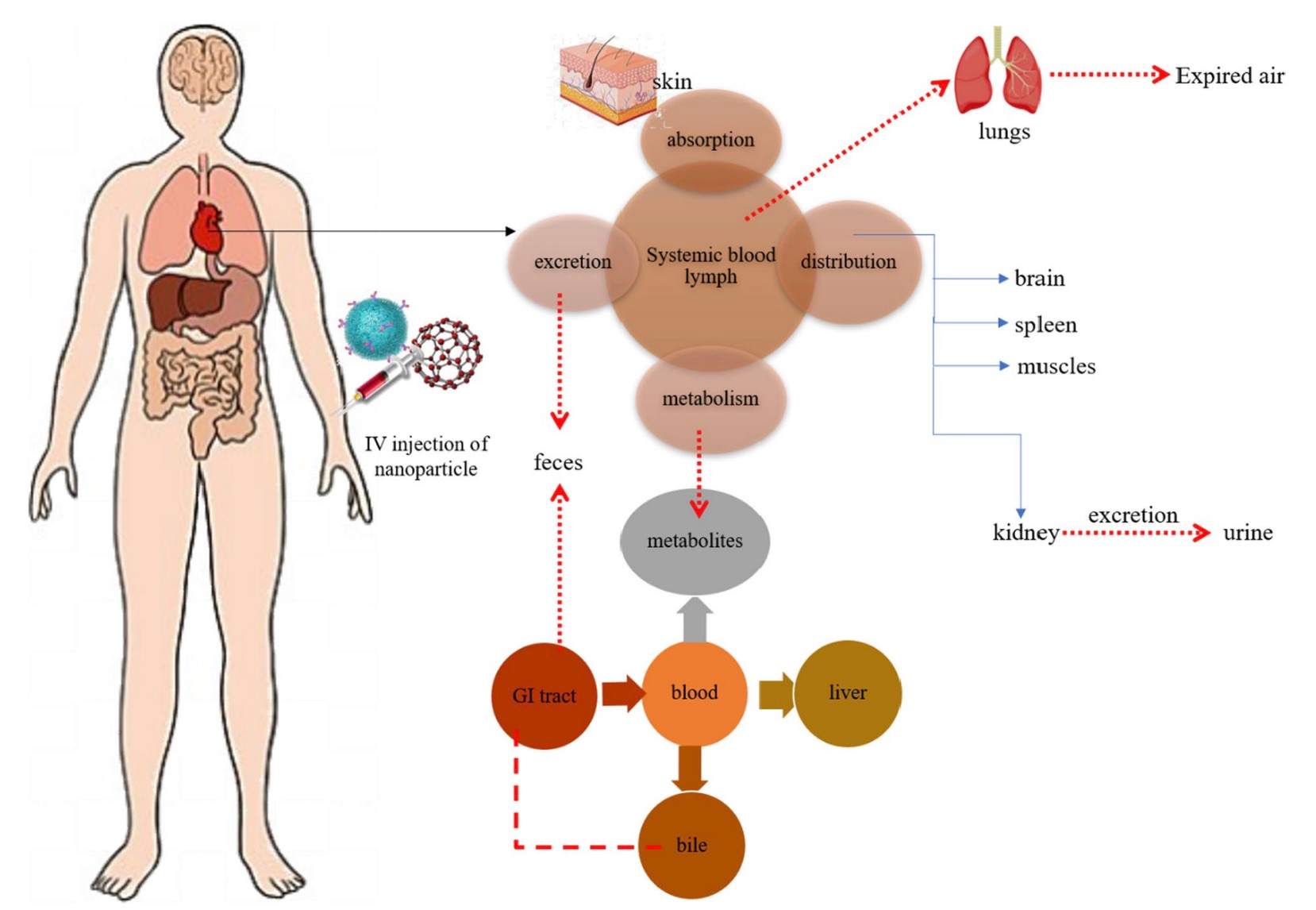 Fig.4 Overall ADME mechanism and fate of nanoparticles.4,5
Fig.4 Overall ADME mechanism and fate of nanoparticles.4,5
Platform
Our platform for DMPK studies offers a wide range of experimental methods and animal models designed to assess the pharmacokinetic and metabolic properties of drug candidates. By combining advanced in vitro and in vivo techniques, we provide detailed insights into a drug's ADME characteristics to support preclinical drug development. In addition to traditional DMPK assessments, we also offer anti-drug antibody (ADA) services, which are crucial for evaluating the immunogenicity of biologic drugs.
In Vitro Experimental Methods
- Microsomal Studies: Liver microsomes are used to evaluate the metabolic stability and enzyme interactions of drug candidates.
- Caco-2 Permeability Assay: Simulates intestinal absorption by studying drug transport across a monolayer of human enterocyte-like cells.
- Plasma Protein Binding Study: Determines the percentage of a drug that is bound to plasma proteins, affecting its pharmacological activity and bioavailability.
- Cytochrome P450 Induction/Inhibition Assay: Measures the impact of drugs on liver enzymes, crucial for understanding drug-drug interactions.
- Enzyme-Linked Immunosorbent Assay (ELISA): The most common method for detecting and quantifying ADA in patient serum. It uses a solid-phase antigen to capture antibodies, followed by detection using enzyme-conjugated secondary antibodies.
- Electrochemiluminescence Immunoassay (ECLIA): A sensitive immunoassay that detects ADA using electrochemiluminescent labels. It offers high sensitivity and specificity, useful for detecting low titers of ADA.
- Radioimmunoassay (RIA): Although less commonly used due to safety concerns, RIA detects ADA using radioactive labels, providing high sensitivity and specificity.
In Vivo Experimental Methods
- Single-Dose and Multiple-Dose Studies: These studies evaluate the kinetics of drug absorption, distribution, metabolism, and elimination after both single and repeated doses.
- Tissue Distribution: Blood, tissue, and organ samples are collected to analyze drug concentration over time and determine bioavailability and tissue-specific accumulation.
- Metabolism Studies: Using advanced technologies like LC-MS/MS, we identify and quantify metabolites, providing information about the metabolic pathways of the drug.
- Pharmacokinetic Modeling: The data from blood sampling and tissue distribution are used to create pharmacokinetic models that predict the drug's behavior in humans.
- Immunization Studies: In vivo studies may involve immunizing animals with the drug or its conjugates to induce ADA production. The subsequent immune response is monitored in blood and tissues.
- Cytokine Profiling: In vivo studies can also involve measuring changes in immune cell activity and cytokine profiles in response to ADA formation. This helps to determine the nature and severity of the immune response.
Applications
- Lead Compound Optimization: DMPK studies help identify and refine lead compounds by assessing their absorption, distribution, metabolism, and excretion properties early in development. This allows for the elimination of poor candidates before clinical trials.
- Dose Prediction: By understanding pharmacokinetic parameters such as half-life, Cmax, and Tmax, DMPK studies help determine optimal dosing strategies for preclinical and clinical phases.
- Metabolism and Toxicity Prediction: Understanding how drugs are metabolized by liver enzymes helps predict potential toxic metabolites and assess whether a drug could cause adverse reactions. This is crucial for avoiding late-stage clinical failures.
- Drug-Drug Interaction Studies: DMPK studies can identify interactions between drugs and other substances, such as co-administered medications or food, which may alter drug absorption or metabolism.
- Bioavailability Enhancement: DMPK studies help optimize drug formulations to improve bioavailability, ensuring drugs are delivered effectively to the intended target in the body.
- Sustained-Release and Targeted Delivery: By studying the pharmacokinetic profile of various formulations, DMPK studies assist in designing sustained-release and targeted drug delivery systems that enhance therapeutic outcomes while minimizing side effects.
Our Advantages
- Expert Team: Experienced scientists with deep knowledge in pharmacokinetics, metabolism, and toxicology, tailored to your therapeutic area.
- Advanced Technologies: Use of state-of-the-art tools like LC-MS/MS and HPLC for precise and reliable data.
- Custom Animal Models: Wide range of rodent and non-rodent models, adaptable to your drug's needs for accurate data.
- Early-Stage Support: Accelerate lead optimization by identifying key pharmacokinetic and metabolic properties early in development.
- Regulatory Expertise: Compliance with FDA/EMA guidelines to support regulatory submissions and accelerate approval.
- Data-Driven Insights: Comprehensive pharmacokinetic profiles and safety assessments to inform decisions.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1. What is the purpose of DMPK studies?
A1: DMPK studies are designed to assess the pharmacokinetics (ADME) of drug candidates, including their absorption, distribution, metabolism, and excretion. These studies help optimize drug formulations, predict human responses, and ensure safety and efficacy.
-
Q2. What animal models are used in DMPK studies?
A2: We primarily use rodent models (mice and rats) but can also utilize non-rodent species based on the drug's characteristics. These models are essential for simulating human pharmacokinetics and providing reliable preclinical data.
-
Q3. How long do DMPK studies take?
A3: Study duration varies depending on the complexity and design. Standard single-dose studies can take 4-6 weeks, while more complex multiple-dose or metabolism studies may require longer periods. We aim to deliver results within your project timeline.
-
Q4: What are the main benefits of conducting DMPK studies early in drug development?
A4: Early DMPK studies help identify potential issues such as poor bioavailability, undesirable metabolism, or drug-drug interactions before clinical trials. This allows for more informed decisions and reduces the risk of costly late-stage failures.
-
Q5: Can DMPK studies predict human pharmacokinetics?
A5: Yes, using animal models that mimic human pharmacokinetics, we can estimate human drug behavior, providing valuable insights for dose selection, potential side effects, and therapeutic efficacy.
References
- Konstandi, Maria, and Elizabeth O Johnson. "Age-related modifications in CYP-dependent drug metabolism: role of stress." Frontiers in endocrinology vol. 14 1143835. 24 May. 2023. https://doi.org/10.3389/fendo.2023.1143835
- Yang, Yi et al. "PBPK Modeling on Organs-on-Chips: An Overview of Recent Advancements." Frontiers in bioengineering and biotechnology vol. 10 900481. 14 Apr. 2022. https://doi.org/10.3389/fbioe.2022.900481
- Pizano-Martinez, Oscar et al. "Anti-Drug Antibodies in the Biological Therapy of Autoimmune Rheumatic Diseases." Journal of clinical medicine vol. 12,9 3271. 4 May. 2023. https://doi.org/10.3390/jcm12093271
- Haripriyaa, M., Suthindhiran, K. Pharmacokinetics of nanoparticles: current knowledge, future directions, and its implications in drug delivery. Futur J Pharm Sci 9, 113 (2023). https://doi.org/10.1186/s43094-023-00569-y
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
